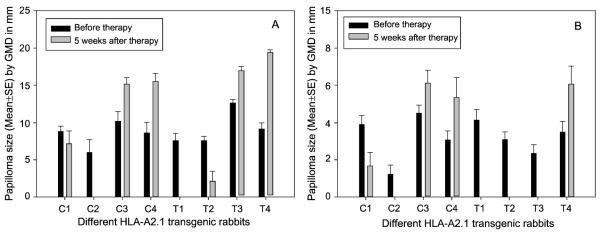
Figure 6.
Papilloma outgrowth in HLA-A2.1 transgenic rabbits after CRPVE1/149-157 epitope DNA therapeutic vaccination. Four outbred and four EIII/JC inbred transgenic rabbits were challenged with wild type CRPV (A) and CRPV E8ATGko mutant (B) at four left and right back sites respectively. Four weeks after DNA challenge, two outbred (C1-C2) and EIII/JC (C3-C4) inbred HLA-A2.1 rabbits were vaccinated with HPV16E7/82-90 epitope DNA vaccines. Two outbred (T1-T2) and two EIII/JC inbred (T3-T4) HLA-A2.1 transgenic rabbits were immunized with CRPVE1/149-157 epitope DNA vaccine at week for three times at a three week interval. Papilloma outgrowth was monitored weekly for twelve weeks. Both CRPVE1/149-157 treated EIII/JC inbred rabbits (four papillomas /per animal, eight tumor sites in total) showed significant reduction or regression of wild type CRPV induced papillomas while papillomas on one of the control rabbits regressed (A). No significant difference was found between these two CRPVE1/149-157 and HPV16E7/82-90 epitope vaccinated outbred rabbits upon challenge with wild type CRPV DNA (Eight tumor sites/ group, P>0.05, unpaired student t test).Two CRPVE1/149-157 epitope DNA vaccinated EIII/JC rabbits were free of CRPVE8ATGko mutant induced papillomas at week 5 after treatment. Taken together, when compared with those in HPV16E7/82-90 vaccinated rabbits challenged with E8ATGko mutant DNA, significantly smaller papillomas were found after CRPVE1/149-157 epitope DNA vaccination (B) (sixteen tumor sites/ group, P<0.05 vs. before therapy, unpaired student t test).