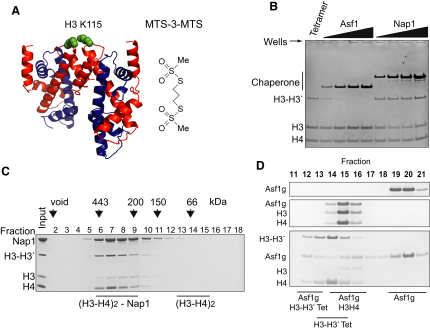
Figure 2.
Directed Sulfhydryl-Reactive Crosslinking Suggests that Nap1 Binds H3 and H4 in Their Tetrameric Conformation
(A) The structure of the (H3-H4)2 tetramer from the nucleosome crystal structure showing the proximity of H3 K115 residues (green spheres) (image created with Pymol, http://www.pymol.org/). Chemical structure of the compound MTS-3-MTS used to crosslink cysteines engineered at position 115 is shown alongside.
(B) The extent of crosslinking under increasing concentrations of cysteine free Nap1 or Asf1 shown by separation of the reaction components by SDS-PAGE. Concentration of chaperone from left to right was 0.25, 0.5, 1.0, and 2.0 μM monomer.
(C) Gel-filtration analysis of the H3 K115C crosslinked tetramer in the presence of Nap1 showing that they remain stably associated under the conditions used.
(D) Globular Asf1 was mixed with noncrosslinked (middle) and crosslinked (bottom) tetramer and the complexes separated by gel-filtration chromatography. The majority of Asf1 does not form a complex with crosslinked tetramers.
See also Figures S1 and S2.