Figure 5.
Nap1 and Vps75 Copurify with Tetrameric H3-H4 and Can Utilize H3 and H4 Trapped in Their Tetrameric Conformation for Their Biological Functions In Vitro
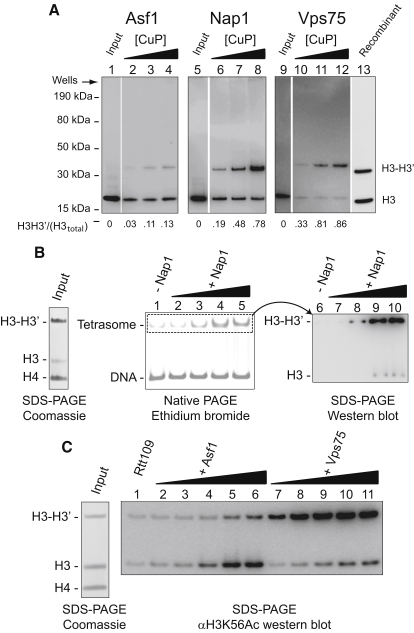
(A) TAP-tagged Asf1, Nap1, and Vps75 were purified from strains expressing H3K115C-myc as the sole source of histone H3. Complexes were subject to crosslinking in the presence of 0.064, 0.25, or 1.0 mM CuP (Asf1, lanes 2–4; Nap1, lanes 6–8; Vps75, lanes 10–12), separated by SDS-PAGE, and immunoblotted within an anti-myc antibody. The intensity of the anti-myc signal was quantified, and the proportion of crosslinked H3 of the total is displayed at the bottom of each lane. Lane 13 contains recombinant partially crosslinked (nontagged) H3 developed with a H3 antibody as a size standard.
(B) (H3-H4)2 tetramer deposition by Nap1. Left: Crosslinked tetramer input stained with Coomassie. This indicates that the majority of histone H3 present is crosslinked. Center: Monotetrasomes were separated from free DNA by native gel electrophoresis. Lane 1, without Nap1; lanes 2, 3, 4, and 5, 0.2, 0.5, 1.0, and 2.0 μM Nap1, respectively. Right: The monotetrasomal bands were excised from the native gel, subjected to SDS-PAGE, and probed by western blotting for H3. Lanes 6–10 correspond to lanes 1–5 from the center panel. This shows that crosslinked tetramers are present in tetrasomes assembled by Nap1.
(C) Vps75 preferentially promotes Rtt109 acetylation of crosslinked tetramer, whereas Asf1 preferentially directs Rtt109 acetylation in favor of noncrosslinked tetramer. Left: Equimolar amounts of crosslinked to noncrosslinked H3 K115C was used as an input. Right: Lane 1, Rtt109 alone (140 nM); lanes 2–6, 60, 80, 100, 120, and 140 nM each of Asf1 and Rtt109, respectively; lanes 7–11, 10, 15, 20, 25, and 30 nM of (Vps75)2-Rtt109 complex, respectively.
See also Figure S4.