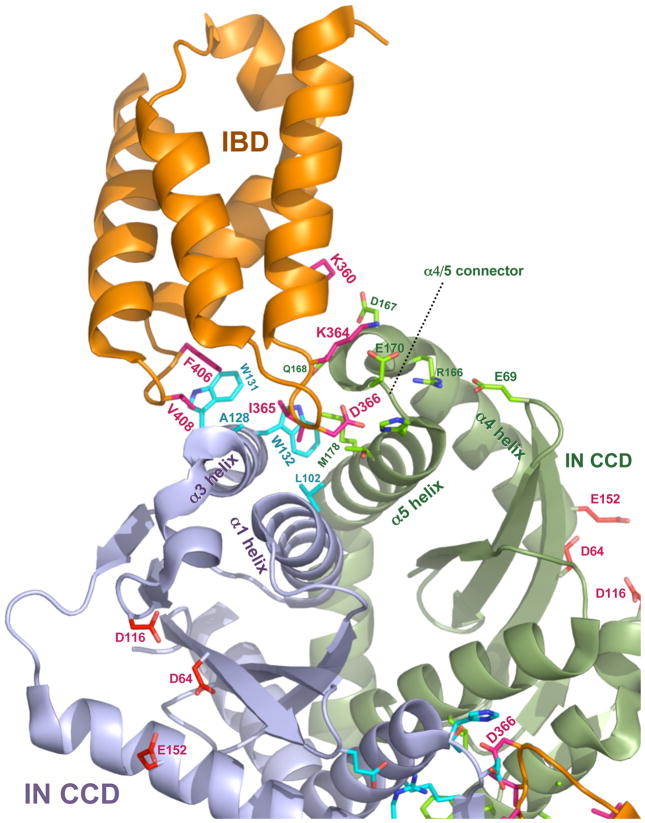
Figure 3. Co-crystal structure of the HIV-1 IN CCD-IBD interface.
The figure was constructed with MacPyMOL from Protein Data Bank file 2BJ4 (www.pdb.org, ref. [13]. LEDGF/p75 contributes most of the amino acid side chains that make direct contact with IN. D366 engages in a pair of essential hydrogen bonds with the main chain amides of E170 and H171 in the alpha-4/5 connector of one IN monomer. Hydrophobic interactions predominate in interactions with the other IN monomer: IN residues W132, W131, A128, L102 form a pocket in the vicinity of IBD residues F406 and V408; this pocket buries IBD residue I365). IN catalytic center resides D64, D116 and E152, the mutation of which produce purely catalytic defects (reviewed in [25]) are shown in the lower half of the figure for each IN monomer. Interaction between the HIV-1 IN NTD and the IBD was previously established by biochemical evidence [53] and Hare et al. have recently solved a co-crystal structure for the LEDGF/p75 IBD complexed with a two-domain fragment of HIV-2 IN (NTD+CCD) [38]. Extensive structural contacts between the IBD and the NTD were identified and characterized [38]. Charged interactions are dominant, with conserved acidic residues in the HIV-2 IN NTD (E6, E10, E13) engaging complementary basic residues in the IBD (K401, R404, R405). Moreover, the NTDs of other lentiviral INs contain the same or closely adjacent glutamic acid residues. Enhancement by LEDGF/p75 of concerted strand transfer activity in vitro was also shown to be impaired by charge-reversing mutation of the basic IBD residues to glutamates in the equine lentivirus (EIAV) IN [38]. This activity could then be partially restored by reciprocal mutation of the acidic IN residues to lysines, verifying that these charged interactions are the main structural feature. See ref. [17] for a recent review of HIV-1 IN structural biology.