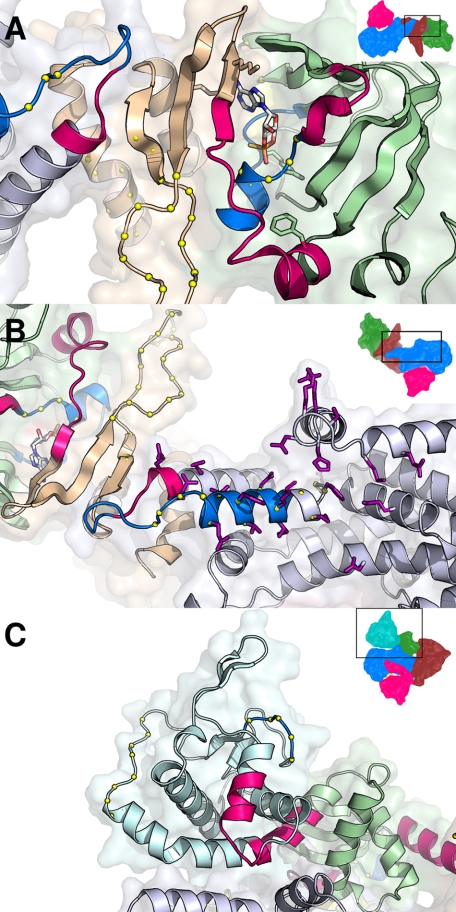
FIGURE 8.
Details of the cAMP-induced conformational changes in Epac2 revealed by DXMS. A, structure of the hinge/switchboard in the (Sp)-cAMP-bound Epac2. The regions highlighted in bright red or blue become respectively more or less exposed upon cAMP binding. The domains are colored as follows: yellow, CBD-A; cyan, DEP; green, CBD-B; brown, REM; pink, RA; blue, GEF. B, the RAP1B binding site. Residues involved in RAP1B binding to the active Epac2 molecule are shown as purple sticks. The RAP1B molecule was removed for clarity. C, the DEP domain of apo-Epac2 with regions of increased solvent accessibility due to cAMP binding colored in red.