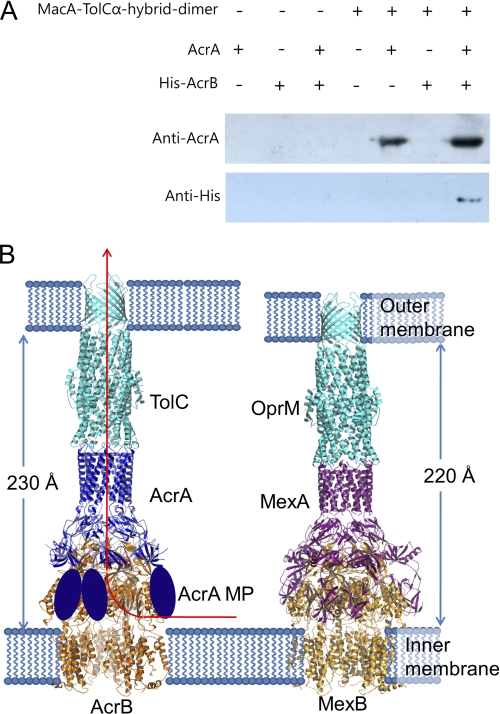
FIGURE 6.
Assembly model for tripartite efflux pumps. A, interaction of TolC α-barrel tip, AcrA, and AcrB. MacA-TolCα hybrid dimer-coupled resin was prepared using CNBr-activated resin (50 μg; GE Healthcare) and MacA-TolCα hybrid dimer protein (1 mg) according to the manufacturer's instruction. AcrA (residues 26–397; 2 mg/ml) and/or the full-length AcrB with C-terminally hexahistidine tag (His-AcrB; 2 mg/ml) were incubated with the coupled resin (20 μl) for 2 h at 4 °C, and then the resin was thoroughly washed with PBS and applied to SDS-PAGE, followed by Western blotting. To detect AcrA and AcrB, anti-AcrA and anti-His antibodies were used, respectively. B, left, the ribbon representation of the TolC3-AcrA6-AcrB3 complex is colored by its components. The TolC trimer (cyan) contacts the funnel stem of the AcrA hexamer (blue), and the AcrB trimer (orange) contacts the funnel mouth of the AcrA hexamer. In particular, the flexible AcrA MP domains (blue ovoid) make a pair and interact with the porter domain of AcrB, accommodating the dynamic structural movement coupled with the proton translocation and the substrate transport. The substrate moving passage is indicated by a red arrow. The complex spans the entire periplasmic space, inner membrane, and outer membrane. The periplasmic part of the ternary complex is 230 Å long. Right, the ribbon representation of the OprM3-MexA6-MexB3 complex. The OprM trimer (cyan), MexA hexamer (violet), and MexB trimer (orange) make a long complex in the same arrangement with the TolC-AcrA-AcrB complex. The MP domains of MexA are shown. The periplasmic part of the complex is 10 Å shorter because the α-hairpin domain of MexA is shorter than that of AcrA.