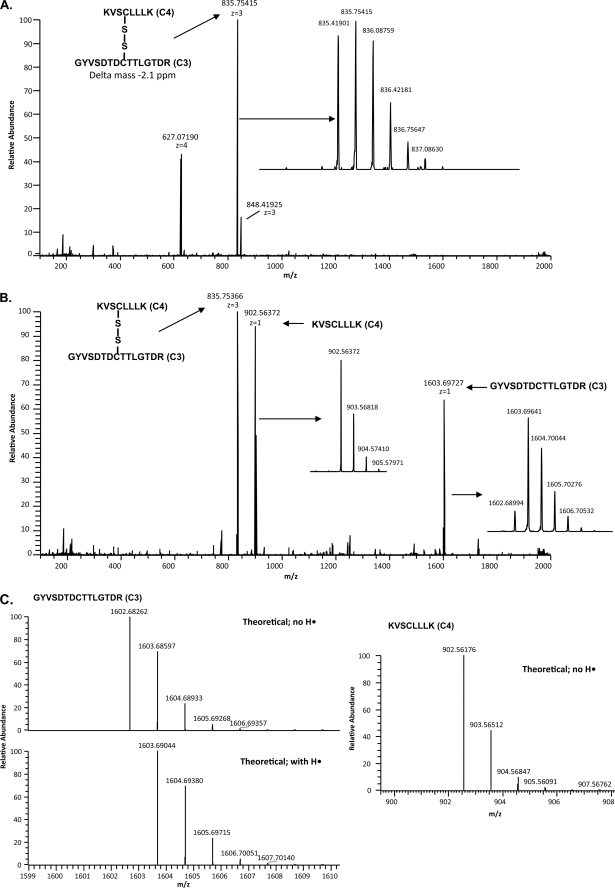
FIGURE 3.
Example of the mass spectrometry analysis of a disulfide-bonded peptide pair using ECD. A, FTICR full scan of the HPLC separation of trypsin-digested SUATM129 protein fraction 57 is shown. The ion with an exact mass corresponding to the C3 peptide and the C4 peptide connected by a disulfide bond is indicated. The isotopic distribution of the peptides is shown in the inset. B, ECD scan triggered from the 835.41901 [M+3H]3+ ion is shown. Experimental conditions were used so that each of the singly charged ECD fragment ions as well as the parent ion were present in the ECD spectra. The singly charged ECD fragment ions add up to the mass of the parent ion. The isotopic distributions of the ECD fragment peptides are shown in the insets. C, ECD fragmentation can produce peptides with variable protonation. The isotopic distribution of the ECD fragment peptides shows that the resulting cysteine sulfurs can be partially protonated (C3) or completely deprotonated (C4) compared with the theoretical distributions.