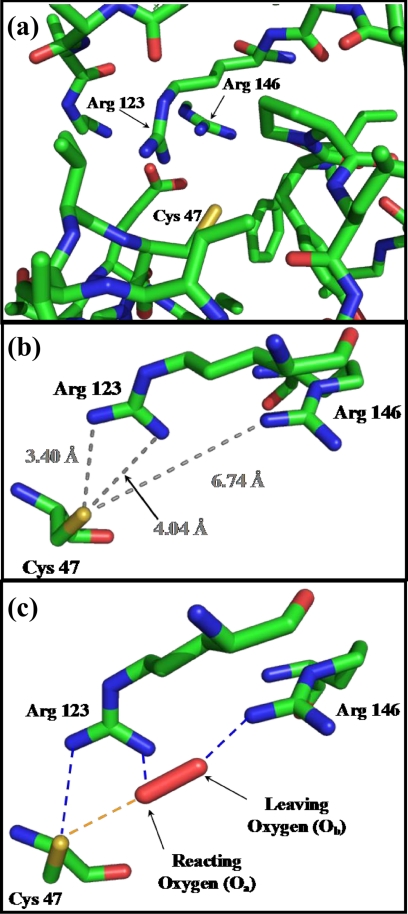
FIGURE 1.
There are two highly conserved arginine residues at the active site of Prx3, which play major roles in its peroxidase activity. a, active site structure of bovine Prx3 (Protein Data Bank code 1ZYE) around its peroxidative cysteine (Cys-47) residue. b, distances of ArgI (Arg-123) and ArgII (Arg-146) nitrogens from the Cp sulfur in the crystal structure of bovine Prx3 (Protein Data Bank code 1ZYE). c, proposed transition structure for the reaction of H2O2 with Prx3 in which ArgI (Arg-123) donates an H-bond to the Cp (Cys-47) sulfur as well as to Oa and ArgII (Arg-146) assists in the protonation of the leaving ObH− moiety.