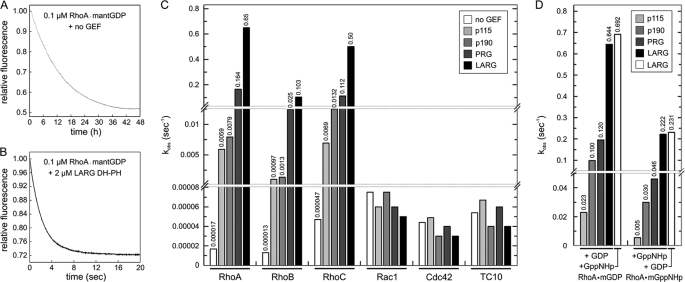
FIGURE 2.
Rho specificity of p115, p190, PRG, and LARG. A and B, DH-PH catalyzes the very slow intrinsic nucleotide exchange reaction by several orders of magnitude. The mantGDP dissociation from 0.1 μm RhoA was monitored after addition of 20 μm unlabeled GDP in the absence (A) and in the presence of 2 μm LARG DH-PH (B). Note the dimension of the x axis, which is in hours in A and in seconds in B, visualizing a rate acceleration of more than 38,000-fold. C, Rho isoform specificity of p115, p190, PRG, and LARG. The observed rate constants (kobs) of both intrinsic and DH-PH-catalyzed reactions of different GTPases were obtained by single exponential fitting of the data. The kobs values were determined using 0.1 μm mantGDP-bound GTPases (RhoA, RhoB, RhoC, Rac1, Cdc42, and TC10) and 20 μm non-fluorescent GDP in the mantGDP dissociation catalyzed by four different DH-PH proteins (2 μm each). D, DH-PH-catalyzed nucleotide exchange is independent of the type of bound nucleotide. GEF-catalyzed mantGDP and mantGppNHp dissociation from RhoA was monitored using 0.1 μm mant-nucleotide-loaded RhoA (RhoA-mantGDP or RhoA-mantGppNHp) and 20 μm non-fluorescent nucleotide (GDP or GppNHp) in the presence of 10 μm DH-PH domain of p115 or p190 or 2 μm DH-PH domain of PRG or LARG. Note that a 5-fold lower concentration of LARG and PRG has been used compared with the experiments with p190 and p115. Moreover, the LARG-catalyzed mant-nucleotide dissociation was measured in the presence of excess amounts of both GDP and GppNHp (white bar). The observed rate constants (kobs) were obtained by single exponential fitting of the data. For convenience, the exact kobs values are given as numbers above the bars in C and D.