Abstract
We have recently described a novel role for pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck) in the induction of ligand-independent epidermal growth factor receptor (EGFR) degradation (Deb TB, Coticchia CM, Barndt R, Zuo H, Dickson RB, and Johnson MD. Am J Physiol Cell Physiol 295: C365–C377, 2008). In the current communication, we explore the probable mechanism by which Pnck induces ligand-independent EGFR degradation. Pnck-induced EGFR degradation is calcium/calmodulin independent and is regulated by cell density, with the highest EGFR degradation observed at low cell density. Pnck is a novel heat shock protein 90 (Hsp90) client protein that can be coimmunoprecipitated with Hsp90. Treatment of Pnck-overexpressing cells with the pharmacologic Hsp90 inhibitor geldanamycin results in enhanced EGFR degradation, and destruction of Pnck. In cells in which Pnck is inducing EGFR degradation, we observed that Hsp90 exhibits reduced electrophoretic mobility, and through mass spectrometric analysis of immunopurified Hsp90 protein we demonstrated enhanced phosphorylation at threonine 89 and 616 (in both Hsp90-α and -β) and serine 391 (in Hsp90-α). Kinase-active Pnck protein is degraded by the proteasome, concurrent with EGFR degradation. A Pnck mutant (T171A) protein with suppressed kinase activity induced EGFR degradation to essentially the same level as wild-type (WT) Pnck, suggesting that Pnck kinase activity is not required for the induction of EGFR degradation. Although EGFR is degraded, overexpression of WT Pnck paradoxically promoted cellular proliferation, whereas cells expressing mutant Pnck (T171A) were growth inhibited. WT Pnck promoted S to G2 transition, but cells expressing the mutant exhibited higher residency time in S phase. Basal MAP kinase activity was inhibited by WT Pnck but not by mutant T171A Pnck protein. Cyclin-dependent kinase (Cdk) inhibitor p21/Cip-1/Waf-1 was transcriptionally suppressed downstream to MAP kinase inhibition by WT Pnck, but not the mutant protein. Collectively, these data suggest that 1) Pnck induces ligand-independent EGFR degradation most likely through perturbation of Hsp90 chaperone activity due to Hsp90 phosphorylation, 2) EGFR degradation is coupled to proteasomal degradation of Pnck, and 3) modulation of basal MAP kinase activity, p21/Cip-1/Waf-1 expression, and cellular growth by Pnck is independent of Pnck-induced ligand-independent EGFR degradation.
Keywords: epidermal growth factor receptor, calmodulin kinase, pregnancy-upregulated nonubiquitous calmodulin kinase, mitogen-activated protein kinase, p21, heat shock protein 90
pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck), a novel member of the calcium/calmodulin kinase family, was previously reported to be expressed in the normal mammary gland during late pregnancy (12) and also in a subset of human breast cancer tissues but not in adjoining benign tissues (12). Pnck is not closely related to the other members of the calmodulin kinase family, sharing only modest sequence similarity with the kinase domain of calmodulin kinase I (13). Expression of Pnck in the mammary gland during late pregnancy was found to be associated with reduced proliferation and terminal differentiation of the gland (12); however, the mechanism underlying this phenotype is unknown. Similarly, the significance of Pnck overexpression in human breast cancer (12) is still unknown.
Epidermal growth factor (EGF) receptor (EGFR), the prototypic member of the ErbB growth factor receptor family (31, 37, 70), is widely expressed in normal tissue and overexpressed/overactivated in a number of malignant tissues, such as brain, lung, renal, and breast cancer (17, 30, 53, 59). Signaling through EGFR (also known as HER-1/ErbB1) is initiated by the binding of its respective ligands, such as EGF, transforming growth factor-α, HB-EGF, etc., followed by homo- or heterodimerization with other members of the ErbB family, namely, ErbB2/HER-2, ErbB3/HER-3, or ErbB4/HER-4. EGFR signaling is vital for normal physiologic processes in several tissues and activates prosurvival and proproliferative biochemical processes, such as MAP and Akt kinase activation (70). The overexpression and overactivation of EGFR is associated with poor outcome in several cancers and, as a consequence, much work has been done to develop EGFR as a therapeutic target in the management of human cancer. EGFR is ubiquitinated by the E3-ubiquitin ligase, c-Cbl (28, 14, 60), followed by degradation by the protea-lysosomal machinery in a ligand-dependent fashion (28), through a mechanism that has been well described. EGFR is also known to be degraded in a ligand-independent manner; however, little information about the mechanisms involved in this process is currently available.
Heat shock protein 90 (Hsp90) is a cellular chaperone that is normally involved in the postsynthesis folding and stabilization of many cellular proteins collectively known as “clients” (40, 43, 44, 46). In cancer cells, several overexpressed, oncogenic proteins, both mutant and normal, depend heavily on the action of Hsp90 for their ability to promote uncontrolled cellular growth and proliferation (39, 64). The chaperone activity of Hsp90 is ATP dependent (18, 38, 42, 44, 46), and pharmacologic Hsp90 inhibitors, such as geldanamycin, 17-AAG, and 17-DMAG, bind to the ATP-binding pocket and inhibit chaperone activity (50). Among the ErbB family members, both newly synthesized and mature ErbB2/HER-2 depend strongly on Hsp90 for chaperoning, and ErbB2 is degraded in cells treated with geldanamycin and its derivatives (34, 66, 67). Less information is available on the role of Hsp90 in the chaperoning of wild-type (WT) EGFR. It has been shown that nascent WT EGFR is degraded in cells treated with geldanamycin (60) while the mature form of the protein does not appear to require Hsp90 chaperoning activity (68). In contrast, mutant EGFR was shown to form a complex with Hsp90 (57) and to be susceptible to geldanamycin treatment (25, 57, 66).
In a recent study we described a novel role for Pnck in the degradation of EGFR and demonstrated that Pnck-mediated EGFR degradation occurs independent of EGFR tyrosine kinase activity and does not require EGFR ligand binding (6). In the present study we have extended this work to explore the mechanism underlying Pnck-induced ligand-independent EGFR degradation. We show that Hsp90 apparently plays a prominent role in maintaining EGFR stability and that this activity is regulated by the Hsp90 phosphorylation state. We explore the potential role of Pnck in the phosphoregulation of Hsp90 activity and propose that Pnck-induced Hsp90 phosphorylation favors EGFR degradation following dissociation of the receptor from the Hsp90 chaperone heterocomplex. Furthermore, we show that the modulation of cellular proliferation by Pnck is not mediated by Pnck-induced EGFR degradation.
MATERIALS AND METHODS
Plasmid constructs, site-directed mutagenesis, generation and propagation of stable transfectant cell clones.
The preparation of the hemagglutinin (HA)-tagged human Pnck cDNA expression construct and the generation of stable HEK-293 cell clones expressing the HA-tagged Pnck protein (and Neo control clones) have been previously described (6). Stable clones were maintained in high-glucose DMEM (catalog no. 10-013 CV, Mediatech) supplemented with 10% heat-inactivated fetal bovine serum (FBS) without G-418 and were used for experiments within a few passages of their isolation. The mutant HA-Pnck (T171A) plasmid was generated by site-directed mutagenesis using a commercial kit according to the manufacturer's protocol (Stratagene) and verified by sequencing. Human embryonic kidney 293 (HEK-293) cell clones expressing the mutant HA-Pnck construct (T171A) were selected using G-418 as previously described for the original construct (6).
Antibodies and pharmacologic inhibitors.
Monoclonal antibodies against human EGFR (Ab-15) and Ab-1 (clone 528) were purchased from NeoMarkers (currently Thermo Scientific). An anti-Pnck rabbit polyclonal antibody (catalog no. AP7097a) was purchased from Abgent. An Hsp90-α/β mAb (F-8) and anti-EGFR pAb (1005) were purchased from Santa Cruz Biotechnology, and the following antibodies were purchased from Stressgen: anti-Hsc70 pAb, anti-p23 pAb, anti-Hsp70 pAb, and anti-Hop (p60) mAb. The anti-Hsp27 mAb was purchased from Assay Designs. The phospho-MAPK pAb and phospho-EF-2 pAb were from Cell Signaling Technology. The β-actin monoclonal antibody was purchased from Sigma, and a pan-ERK mAb was from Pharmingen. The following pharmacologic inhibitors were purchased from Biomol (now Enzo Life Sciences): MG-132, BAPTA-AM, W-7, and geldanamycin.
Cell lysis, Western blot analysis, immunoprecipitation, and immunokinase assay.
Cell lysis, Western blot analysis, immunoprecipitation, and immunokinase procedures were conducted as previously described (6). Briefly, cells were lysed in a buffer containing 1% Triton X-100 (8) and the protein concentration in the lysates was determined by BCA Protein Assay Reagent (Pierce). Equal amounts of total protein were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membrane, immunoblotted with the respective primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies, and finally visualized using enhanced chemiluminescence reagent and X-ray film. For immunoprecipitation, precleared lysates were incubated with primary antibodies followed by GammaBind G Sepharose beads (GE Healthcare) which were washed three times in lysis buffer. Immunokinase assays were performed as previously described (6).
Hsp27 short hairpin RNA lentivirus preparation and cell infection.
Hsp27 short hairpin RNA (shRNA) lentiviral constructs were purchased from Open Biosystems Thermo Scientific (Huntsville, AL). Plasmid DNA was prepared using the HiSpeed Plasmid Midi Kit (Qiagen, Valencia, CA) and checked for quality and quantity by agarose gel electrophoresis and a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). HEK-293T cells were cotransfected with the shRNA constructs, together with pHR′Δ8.2 and pCMV-VSV-G plasmids using FuGENE 6 (Roche, Indianapolis, IN). Viral supernatants were collected 48 h after transfection, and viral titer was determined by limiting dilution. Experimental cells were infected with viral supernatants supplemented with polybrene (6 μg/ml). After 24 h the virus was removed and replaced with regular media.
Mass spectrometry.
Low-density monolayer cultures of Neo, HA-Pnck, and T171A HA-Pnck HEK-293 cells were lysed in lysis buffer (8), and protein concentrations were determined. Hsp90 was then immunoprecipitated from lysate samples adjusted to contain equal amounts of total protein using an anti-Hsp90 mAb (Santa Cruz Biotechnology). Five percent of each immunoprecipitate was reserved for subsequent SDS-PAGE and immunoblot analysis to verify the specificity of the immunoprecipitation. The remaining 95% of each sample was resolved by SDS-PAGE and stained with Coomassie Blue G-250. Mass spectrometric analysis of immunoprecipitated Hsp90 was performed using a two-step process. 1) First, the protein bands of interest were manually excised from the gel, transferred to the wells of a Montage filter plate (Millipore), destained with 50% acetonitrile in 25 mM ammonium bicarbonate, dehydrated with acetonitrile for 5 min, and vacuum dried. The gel pieces were then rehydrated with 15 μl of ammonium bicarbonate: acetonitrile (25 mM: 10%) supplemented with trypsin (5 ng/μl, Promega, Madison, WI) and subjected to in-gel trypsinization by incubation at 37°C for 16 h. After digestion, the tryptic peptides were extracted in 0.1% trifluoroacetic acid-80% acetonitrile and dried under vacuum. 2) Second, the resultant peptide samples were subjected to nano-LC-MS/MS analysis using a nano Acquity UPLC system (Waters) interfaced with a QSTAR ELITE mass spectrometer (Applied Biosystems). The samples were reconstituted in solvent A (98% acetonitrile, 2% water, and 0.1% formic acid). The nanoflow UPLC system was used to deliver sample at a flow rate of 300 nl/min, and chromatographic separation was accomplished using a nano Acquity UPLC BEH C18 column (Waters). Sequential elution of peptides was accomplished using a linear gradient from 5% solvent A to 60% solvent B (98% acetonitrile, 2% water and 0.1% formic acid) over 60 min. The mass spectrometer was operated in positive ion mode with a resolution of 10,000–12,000 at full width half-maximum for the Q STAR Elite using a source temperature of 200°C. For MS/MS analysis, survey scans were acquired from m/z 300 to 1,500 with up to three precursors selected for MS/MS from m/z 100 to 2,000 using dynamic exclusion, and rolling collision energy was used to promote fragmentation.
Cell proliferation assay.
Cell proliferation assays were conducted by plating the cells in 60-mm BD Biocoat dishes at low densities in complete medium. The cells were allowed to grow for the indicated periods, after which they were trypsinized and counted using a hemocytometer.
Cell cycle analysis.
Cell cycle analysis was performed using propidium iodide staining of DNA followed by flow cytometry-based analysis of distribution of cells at the different phases of the cell cycle. In brief, cells were plated at low cell densities and synchronized at either the S phase by the addition of thymidine (5 mM) for 48 h, or the G2 phase by nocodazole treatment (50 ng/ml) for 16 h, then washed twice and released by culture in complete medium. The cells were trypsinized and fixed in 75% ethanol at the indicated time points after release, stained with propidium iodide, and analyzed by the Flow Cytometry Shared Resource at the Lombardi Comprehensive Cancer Center.
Total RNA isolation and quantitative real-time polymerase chain reaction.
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) and the RNeasy kit (Qiagen) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed in a total volume of 20 μl using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) per the manufacturer's instruction and subsequently diluted to 500 μl with sterile water. Quantitative, real-time PCR was performed in 20-μl reactions using 1× SYBR green PCR master mix (Applied Biosystems), 125 nM each of forward and reverse primers, and 5 μl of diluted cDNA, using an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems) for 40 cycles (95°C for 15 s, 60°C for 1 min) following initial 10-min incubation at 95°C. The fold change in expression of transcripts was calculated using the ΔΔCt method (where Ct is cycle threshold), with the ribosomal protein 36B4 mRNA as the internal control. The primer sequences used were as follows: p21 forward, 5′-TGGAGACTCTCAGGGTCGAAA-3′; p21 reverse, 5′-GGCGTTTGGAGTGGTAGAAAT-3′; 36b4 forward, 5′-GTGTTCGACAATGGCAGCAT-3′; 36b4 reverse, 5′-GACACCCTCCAGGAAGCGA-3′.
RESULTS
Pnck-induced EGFR degradation is calcium/calmodulin independent.
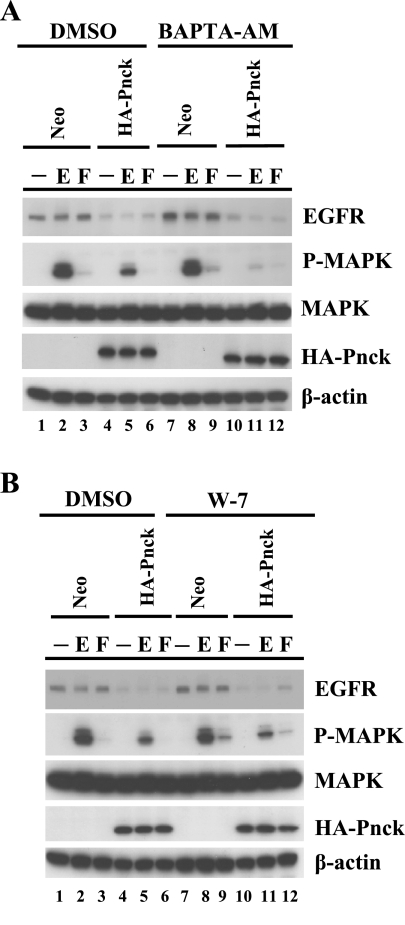
Based on analysis of its primary amino acid sequence, Pnck was previously classified as a novel calcium/calmodulin kinase with a calmodulin binding regulatory domain at the COOH terminus (13). To determine whether the ligand-independent EGFR degradation activity of Pnck that we previously described (6) is dependent on calcium and calmodulin, the known allosteric regulators for activation of a typical calmodulin kinase, we pretreated both Neo and HA-Pnck cells with a specific intracellular calcium chelator, BAPTA-AM, and a pharmacologic calmodulin antagonist, W-7 (Fig. 1, A and B). Stable Neo and HA-Pnck HEK-293 cells were pretreated with either vehicle (DMSO) or inhibitors and subsequently stimulated without or with EGF. EGFR, HA-Pnck, and activated MAP kinase levels in cell lysates were monitored by immunoblotting. EGFR levels were significantly lower in vehicle (DMSO)-treated HA-Pnck cells compared with Neo cells, consistent with the Pnck-mediated ligand-independent EGFR degradation previously observed (6) [Fig. 1A, Western blot (WB): EGFR, lanes 1 and 4, and Fig. 1B, WB: EGFR, lanes 1 and 4]. Receptor levels were further suppressed through ligand-dependent EGFR degradation following EGF stimulation (Fig. 1A, WB: EGFR, lanes 2 and 5, and Fig. 1B, WB: EGFR, lanes 2 and 5). EGF-induced MAP kinase activation was used as a functional readout of reduced functional EGFR levels through ligand-independent EGFR degradation which showed an inhibition in MAP kinase activity in vehicle-treated HA-Pnck-overexpressing cells compared with Neo cells under the same treatment [Fig. 1A, WB: phospho(P)-MAPK, lanes 2 and 5, and Fig. 1B, WB: P-MAPK, lanes 2 and 5]. Treatment with BAPTA-AM did not appear to have any effect on the Neo cells compared with the vehicle-treated Neo cells, with or without EGF stimulation (Fig. 1A, WB: EGFR, lanes 1 and 2 and lanes 7 and 8; WB: P-MAPK, lanes 2 and 8) and failed to restore EGFR levels in the HA-Pnck cells (Fig. 1A, WB: EGFR, lanes 4 and 10). In fact, EGF-dependent MAP kinase activation was inhibited in the HA-Pnck cells after treatment with BAPTA-AM (Fig. 1A, WB: EGFR, lanes 5 and 11). Treatment of HA-Pnck-overexpressing cells with W-7 also did not result in any appreciable change in ligand-independent EGFR expression (Fig. 1B, WB: EGFR, lanes 4 and 10) and EGF-induced MAP kinase activity (Fig. 1B, WB: P-MAPK, lanes 5 and 11), implying that Pnck-regulated ligand-independent EGFR degradation and EGF-induced MAP kinase activation are calmodulin independent. We also examined the effect of both inhibitors (BAPTA-AM and W-7) on serum-induced MAP kinase activation in Neo and HA-Pnck cells. Acute treatment with serum in vehicle-treated Neo and HA-Pnck cells induced very modest MAP kinase activation (Fig. 1A, lanes 3 and 6), and this induction was slightly enhanced by BAPTA-AM treatment (Fig. 1A, lanes 9 and 12). W-7 treatment also slightly increased the serum-induced MAP kinase activation in Neo cells (Fig. 1B, lanes 3 and 9) and in the HA-Pnck cells (Fig. 1B, lanes 6 and 12), indicating a calcium/calmodulin regulation of serum-induced MAP kinase activity in both Neo and HA-Pnck cells. Together, these data suggest that Pnck requires neither calcium nor calmodulin to be able to induce ligand-independent EGFR degradation.
Fig. 1.
Pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck)-induced ligand-independent epidermal growth factor (EGF) receptor (EGFR) degradation is calcium and calmodulin independent. A: Pnck-induced EGFR degradation is calcium independent. Human embryonic kidney (HEK)-293 cells stably expressing Neo (lanes 1–3 and 7–9) or hemagglutinin (HA)-Pnck (lanes 4–6 and 10–12) were serum starved for 3 h and incubated with either vehicle (DMSO) (lanes 1–6) or 10 μM BAPTA-AM for 30 min (lanes 7–12), then stimulated without (lanes 1, 4, 7, and 10) or with (lanes 2, 5, 8, and 11) 10 nM EGF for 5 min (E) or with 10% heat-inactivated serum (lanes 3, 6, 9, and 12) for 10 min (F). B: Pnck-induced EGFR degradation is calmodulin independent. HEK-293 cells stably expressing Neo (lanes 1–3 and 7–9) or HA-Pnck (lanes 4–6 and 10–12) were serum starved for 3 h and incubated with either vehicle (DMSO) (lanes 1–6) or 30 μM W-7 (lanes 7–12) for 45 min, and then stimulated without (lanes 1, 4, 7, and 10) or with (lanes 2, 5, 8, and 11) 10 nM EGF for 5 min (E) or with 10% heat-inactivated serum (lanes 3, 6, 9, and 12) for 10 min (F). For both A and B, cells were lysed and equal amounts of total protein were immunoblotted for EGFR [Western blot (WB): EGFR], HA-Pnck (WB: HA-Pnck), phospho-MAPK (WB: P-MAPK), MAP kinase (WB: MAPK), and β-actin (WB: β-actin). A representative example of three independent experiments is presented.
Elongation factor 2 (EF-2) is phosphorylated by EF-2 kinase, a calcium/calmodulin kinase that is negatively regulated by calcium/calmodulin. Growth factor-induced mobilization of calcium/calmodulin inactivates EF-2 kinase (49), resulting in inhibition in EF-2 phosphorylation, and so we monitored P-EF-2 levels to verify the effectiveness of the BAPTA-AM and W-7 treatments. Treatment of Neo cells with EGF causes a dramatic reduction in P-EF-2 levels (Supplemental Fig. S1, WB: P-EF-2, lanes 2 or 10; Supplemental Material for this article is available online at the Journal website), and this suppression is strongly blunted by treatment with BAPTA-AM (lane 6), or W-7 (lane 14), demonstrating that both of these agents are able to permeate the Neo cells and block calcium/calmodulin activity. Interestingly, expression of HA-Pnck itself reduces the EGF-induced suppression of P-EF-2 levels (WB: P-EF-2, lanes 4 or 12), but treatment with BAPTA-AM and W-7 enhances this effect, demonstrating the efficacy of the treatments (lanes 8 and 16). Collectively, these data indicate that Pnck-induced ligand-independent EGFR degradation is not regulated by either calcium or calmodulin.
Pnck enhances the degradation of EGFR caused by treatment with the Hsp90 inhibitor geldanamycin and is itself an Hsp90 client.
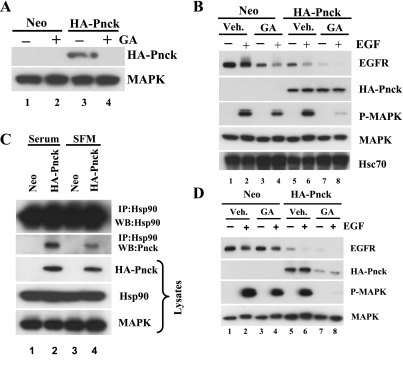
In contrast to the extensive studies of ligand-dependent EGFR degradation that have elucidated the central role played by c-Cbl, an E3-ubiquitin ligase, little mechanistic information is available in the literature regarding ligand-independent EGFR degradation. Treatment of cells with the pharmacologic Hsp90 inhibitor geldanamycin was previously shown to result in the degradation of nascent EGFR but not that of mature membrane integrated WT EGFR (52, 60). Given these data, and the apparent role of Pnck in EGFR degradation, we hypothesized that Pnck's activity might be linked with Hsp90 chaperone function and that Pnck's proximity to Hsp90 as a client might allow it to regulate Hsp90 function. To determine whether Pnck is itself an Hsp90 client, we treated both Neo and HA-Pnck HEK-293 cells with or without the specific pharmacologic Hsp90 inhibitor geldanamycin, prepared cell lysates, and examined the levels of Pnck in the lysates. As hypothesized, geldanamycin treatment resulted in the almost complete loss of HA-Pnck protein, possibly due to proteasomal degradation of the protein (6) (Fig. 2A, lane 4), strongly suggesting that Pnck is indeed a novel Hsp90 client protein. We next set out to test whether Pnck induces enhanced EGFR degradation in geldanamycin-pretreated HA-Pnck cells and to determine whether this enhanced EGFR degradation is reflected in the suppression of EGF-induced MAP kinase activation. Neo and HA-Pnck cells were serum starved, preincubated in the presence or absence of geldanamycin, and then stimulated with or without EGF. As a control for geldanamycin activity, we first analyzed the level of Hsc70 in the lysates since this protein is known to be transcriptionally upregulated in response to geldanamycin. As expected, Hsc70 levels were elevated after geldanamycin treatment in both Neo and HA-Pnck cells (Fig. 2B, WB: Hsc70, lanes 3 and 4 and lanes 7 and 8). Next, the levels of EGFR, HA-Pnck, and phospho-MAPK were analyzed in the cell lysates, and as demonstrated in Fig. 2B, treatment of Neo cells with geldanamycin significantly reduced unliganded EGFR levels compared with vehicle-treated Neo cells (Fig. 2B, WB: EGFR, lanes 1 and 3). Treatment of the cells with EGF resulted in the suppression of EGFR levels still further due to ligand-induced degradation of the receptor (Fig. 2B, WB: EGFR, lanes 3 and 4). As previously reported (6), because of Pnck-induced EGFR degradation, EGFR levels were significantly lower in the HA-Pnck cells, with levels similar to those found in the geldanamycin-treated Neo cells (Fig. 2B, WB: EGFR, lanes 3 and 5) and unliganded EGFR levels were dramatically further suppressed in the HA-Pnck cells treated with geldanamycin (Fig. 2B, WB: EGFR, lane 7). Thus, enhanced ligand-independent EGFR degradation was observed in geldanamycin-treated HA-Pnck cells. Treatment of the geldanamycin-pretreated HA-Pnck cells with EGF resulted in almost undetectable levels of EGFR due to the additional effects of ligand-induced receptor degradation (Fig. 2B, and WB: EGFR, lane 8). The synergy between Pnck and geldanamycin in inducing ligand-independent EGFR degradation was reflected in the almost complete suppression of EGF-induced MAP kinase activity in geldanamycin-treated HA-Pnck cells (Fig. 2B, WB: P-MAPK, lane 8). Although we do not rule out a possible contributory role from the suppression of another Hsp90 client in the MAP kinase signaling pathway (such as Raf-1 or MEK-1) for this almost total suppression of EGF-induced MAP kinase activation (Fig. 2B, WB: P-MAPK, lane 8), we believe that this is principally the result of the synergistic effects of Pnck expression and geldanamycin treatment on EGFR levels, prior to EGF stimulation.
Fig. 2.
Pnck is a heat shock protein 90 (Hsp90) client protein, and Pnck-induced ligand-independent EGFR degradation is enhanced synergistically by geldanamycin-induced EGFR degradation. A: Pnck is degraded in cells treated with geldanamycin. Duplicate dishes of Neo (lanes 1 and 2) and HA-Pnck (lanes 3 and 4) HEK-293 cells were incubated without (lanes 1 and 3) or with 1 μM geldanamycin (GA) (lanes 2 and 4) in serum-containing medium for 4 h. Lysates were prepared, and samples containing equal amounts of total protein were separated on an SDS-PAGE gel, transferred, and immunoprobed using the anti-HA mAb (WB: HA-Pnck) and with an antibody against MAP kinase (loading control). B: Pnck synergizes with geldanamycin in the absence of serum. Four dishes each of Neo (lanes 1–4) and HA-Pnck (lanes 5–8) HEK-293 cells were serum starved overnight and incubated with either DMSO (lanes 1 and 2 and lanes 5 and 6) [vehicle (Veh)] or 1 μM geldanamycin (lanes 3 and 4 and lanes 7 and 8). Cells were treated without (lanes 1, 3, 5, and 7) or with (lanes 2, 4, 6, and 8) 10 nM EGF for 5 min. Equal amounts of total protein from each dish were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: HA-PNCK), P-MAPK (WB: P-MAPK), MAPK (WB: MAPK), and heat shock cognate 70 (WB: Hsc70). C: reduced association of Pnck and Hsp90 in the absence of serum. Lysates prepared from Neo and HA-Pnck HEK-293 cells grown overnight in serum containing medium (lanes 1 and 2) or in serum-free medium (SFM; lanes 3 and 4) were immunoprecipitated by anti-Hsp90 mAb, after which the immunoprecipitates were immunoblotted for HA-Pnck expression (IP: Hsp90, WB: Pnck) and for Hsp90 expression (IP: Hsp90, WB: Hsp90). Input lysates were also immunoprobed for levels of Pnck (WB: HA-Pnck), Hsp90 (WB: Hsp90), and MAP kinase (as a loading control) (WB: MAPK). D: synergistic suppression of EGFR levels by Pnck and geldanamycin in presence of serum. Four dishes each of Neo (lanes 1–4) and HA-Pnck (lanes 5–8) HEK-293 cells were preincubated with either DMSO (lanes 1 and 2 and lanes 5 and 6) (Veh) or 1 μM geldanamycin (lanes 3 and 4 and lanes 7 and 8) in medium containing serum. Cells were treated without (lanes 1, 3, 5, and 7) or with (lanes 2, 4, 6, and 8) 10 nM EGF for 5 min. Equal amounts of total protein from each dish were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: HA-Pnck), P-MAPK (WB: P-MAPK), and MAPK (WB: MAPK). A representative example of multiple experiments is presented.
Interestingly, when we examined the effects of geldanamycin on HA-Pnck in this experiment, we did not observe significant suppression of HA-Pnck levels (Fig. 2B, WB: HA-Pnck, lanes 5–8), in stark contrast to the pronounced geldanamycin-induced degradation of HA-Pnck previously observed (Fig. 2A, WB: HA-Pnck, lane 4). This was a consistent and puzzling finding, and on examination of the design of the two experiments we found that the only difference (Fig. 2, A and B) was that the geldanamycin was added to the cells in the presence of serum in the first experiment (Fig. 2A), but only after serum starvation in the second experiment (Fig. 2B). This led us to hypothesize that HA-Pnck is associated with Hsp90 in the presence of serum, but that during serum starvation, the HA-Pnck protein largely dissociates from the Hsp90 such that when the geldanamycin subsequently interacts with the Hsp90 ATP-binding pocket, the HA-Pnck is not available for degradation. To examine this possibility, we immunoprecipitated Hsp90 from HA-Pnck cells in the presence and absence of serum and observed that serum starvation dramatically reduces the association of HA-Pnck with Hsp90 [Fig. 2C, immunoprecipitate (IP): Hsp90, WB: Pnck, lanes 2 and 4]. We repeated the synergy experiment conducted in Fig. 2B this time in the presence of serum and observed that HA-Pnck was significantly degraded in HA-Pnck cells as expected (Fig. 2D, WB: HA-Pnck, lanes 7 and 8). Despite the geldanamycin-induced loss of Pnck, it is interesting to note that the synergy between geldanamycin and HA-Pnck for the induction of unliganded EGFR degradation was maintained and EGFR levels are suppressed as observed in experiment 2B (Fig. 2D, WB: EGFR, lanes 3 and 7). Thus, although the HA-Pnck is itself being degraded it is able to suppress EGFR levels and act synergistically with pharmacologic Hsp90 inhibition to inhibit EGF-induced MAP kinase activity (Fig. 2D, WB: P-MAPK, lane 8). One possible explanation for this apparent paradox may relate to the observation that, even though Pnck levels are suppressed by the treatment with geldanamycin under these conditions, the protein is still readily detectable (Fig. 2D, WB HA-Pnck lanes 7 and 8) and it may be that sufficient protein still remains to synergistically suppress EGFR levels.
Pnck expression suppresses the level of the ATP-independent small heat shock chaperone Hsp27; however, this suppression does not explain the Pnck-induced suppression of EGFR levels.
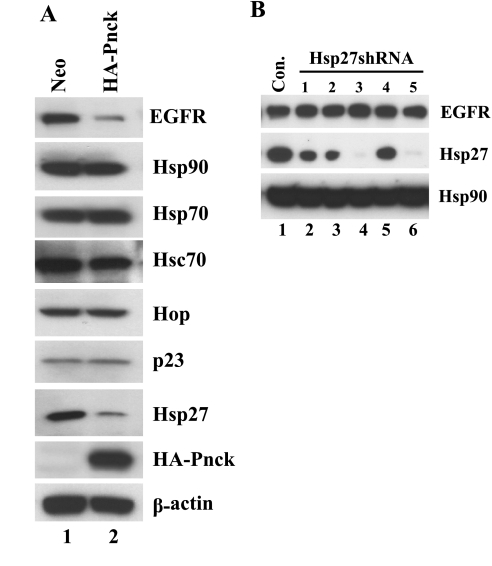
Pnck's association with Hsp90 and the combined effect of Pnck and geldanamycin suppressing EGFR levels suggested that Pnck might be impacting the ATP-dependent Hsp90 chaperone complex. We were also intrigued by a recent report in which small heat shock protein 27 (Hsp27), an ATP-independent chaperone, was shown to modulate geldanamycin sensitivity (33). In this report, geldanamycin sensitivity was significantly enhanced by inhibition of Hsp27 expression by short interfering RNA (siRNA)-mediated knockdown. We argued that since Pnck's effect was similar to that of geldanamycin, it might also downregulate Hsp27 levels, or the levels of other components of the chaperone complex, and thereby enhance geldanamycin's effect on EGFR degradation. We therefore analyzed the expression of a panel of chaperone proteins, such as Hsp90, Hsp70, Hsc70, Hop, p23, and Hsp27, in both Neo and HA-Pnck HEK-293 cells. Interestingly, although the levels of most of the chaperone proteins studied were not different between Neo and HA-Pnck cells (Hsp70, Hsc70, Hop, and p23), the level of Hsp27 protein was significantly (P < 0.005) reduced in the HA-Pnck cells by >50% of the level in the Neo cells (Fig. 3A). To directly test the role that Hsp27 downregulation might play in Pnck-mediated EGFR degradation, parental HEK-293 cells were transiently transduced with several Hsp27-targeting shRNA lentiviral constructs. Cell lysates were then prepared and analyzed for Hsp27 and EGFR levels (Fig. 3B). Interestingly, although several of the shRNA constructs were able to efficiently suppress Hsp27 levels (shRNA 3 and 5, Fig. 3B, lanes 4 and 6), EGFR levels in the cells were unaltered by transduction with any of the constructs (Fig. 3B, WB: EGFR). This suggests that although Hsp27 is downregulated by Pnck, downregulation of Hsp27 alone is not sufficient to mimic Pnck-induced EGFR degradation.
Fig. 3.
Pnck expression suppresses the level of the ATP-independent small heat shock chaperone Hsp27; however, this suppression does not explain the Pnck-induced suppression of EGFR levels. A: Hsp27 levels are suppressed by Pnck. Stable Neo (lane 1) and HA-Pnck (lane 2) HEK-293 cells were plated at low cell density and allowed to grow for 48 h. Cells were lysed, and equal amounts of protein were immunoblotted for EGFR, HA-Pnck, β-actin, Hsp90, Hsp70, Hsc70, Hop (p60), p23, and Hsp27 expression. The experiment was conducted thrice with essentially identical results. B: short hairpin RNA (shRNA)-mediated knockdown of endogenous Hsp27 expression does not result in suppression of EGFR levels. Parental HEK-293 cells were infected with either control virus (lane 1) or one of several Hsp27 shRNA lentiviruses (lanes 2–6) in complete medium using polybrene. Medium was removed after 24 h, and the cells were supplemented with fresh medium. Cells were lysed after an additional 24 h, and equal amounts of total lysate were immunoblotted for EGFR (WB: EGFR), Hsp27 (WB: Hsp27), and Hsp90 (WB: Hsp90) expression.
The Pnck-induced ligand-independent suppression of EGFR levels is cell density dependent.
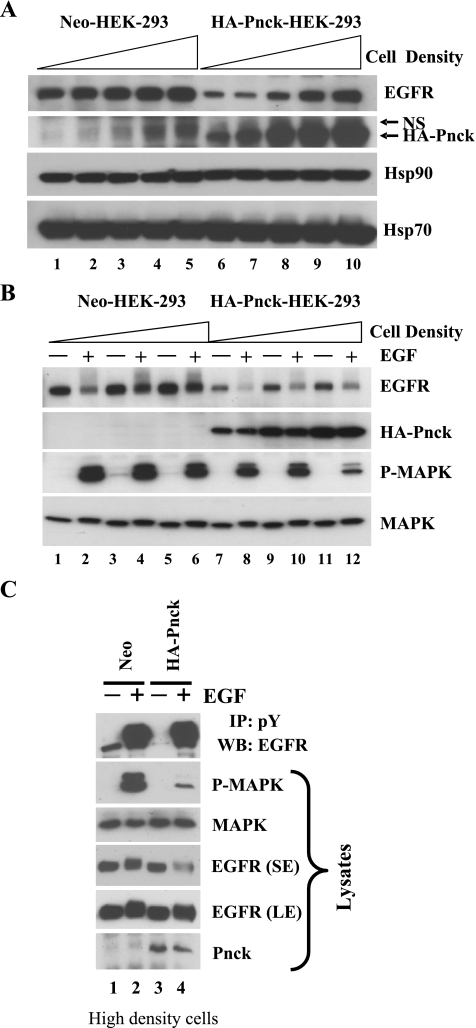
While conducting these experiments, we repeatedly observed that the degree of EGFR level suppression produced by Pnck varied from experiment to experiment depending on cell density at the time of harvest. To explore this phenomenon, both Neo and HA-Pnck cells were plated at different cell densities starting at a very low plating density of 10,000 cells/cm2, and increasing in twofold increments up to 200,000/cm2. Cells were lysed after 48 h and EGFR levels were analyzed in the lysates. As can be seen in Fig. 4A, suppression of EGFR levels by HA-Pnck was strongest at the lowest cell density, and this suppression became weaker with increasing cell density so that EGFR levels in the HA-Pnck cells approached those in the Neo cells at the highest cell density (Fig. 4A, WB: EGFR, lanes 6–10). In contrast, there was only a very modest effect of cell density on EGFR levels in the Neo cells. Interestingly, HA-Pnck levels followed a similar pattern to that of EGFR in the HA-Pnck cells, with a gradual increase in protein level with cell density (Fig. 4A, WB: HA-Pnck). We have previously shown that exogenous Pnck mRNA levels are not significantly altered with changing cell density and that Pnck protein undergoes proteasomal degradation in cells in which it is promoting EGFR degradation (6). Thus, the increase in Pnck levels might result from suppression of Pnck proteasomal degradation at high cell density. We also noticed that there was a slight but consistent decrease in the electrophoretic mobility of Hsp90 at all cell densities in the HA-Pnck cells, suggesting that Hsp90 is posttranslationally modified in these cells. (Fig. 4A, WB: Hsp90, lanes 6–10). In contrast to Hsp90, Hsp70 mobility did not appear to be altered in either Neo or HA-Pnck cells, irrespective of cell density, implying that this effect on protein motility was specific for Hsp90. Hsp70 protein levels, however, were slightly increased in HA-Pnck cells at all densities. Overall, these data indicated that the induction of ligand-independent EGFR degradation by Pnck is negatively modulated with increasing cell density, with the highest suppression of EGFR levels at the lowest cell density.
Fig. 4.
Pnck-induced ligand-independent suppression of EGFR levels is cell density dependent. A: cell density negatively regulates EGFR degradation in HEK-293 Pnck cells. Stable Neo (lanes 1–5) and HA-Pnck (lanes 6–10) HEK-293 cells were plated with increasing cell densities: 10,000 cells/cm2 (lanes 1 and 6), 20,000 cells/cm2 (lanes 2 and 7), 50,000 cells/cm2 (lanes 3 and 8), 100,000 cells/cm2 (lanes 4 and 9), and 200,000 cells/cm2 (lanes 5 and 10) and allowed to grow for 48 h. Cells were lysed, and equal amounts of total protein were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: HA-Pnck), Hsp90 (WB: Hsp90), and Hsp70 (WB: Hsp70). A nonspecific band (NS) appears above the HA-Pnck band. B: effect of cell density on EGF-induced MAP kinase activation. Neo (lanes 1–6) and HA-Pnck (lanes 7–12) HEK-293 cells were plated at increasing cell densities in duplicate plates and grown for 24 h, followed by serum starvation for 12 h. Cells then were treated without (lanes 1, 3, 5, 7, 9, and 11) or with 10 nM EGF (lanes 2, 4, 6, 8, 10, and 12) for 5 min and lysed. Lysates were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: HA-Pnck), P-MAPK (WB: P-MAPK), and MAPK (WB: MAPK) expression. Experiments were repeated multiple times, and a representative experiment is presented. C: EGF-induced tyrosine phosphorylation of EGFR at high cell density in Neo and Pnck cells. Neo (lanes 1 and 2) and HA-Pnck cells (lanes 3 and 4) were plated at high cell density and grown for 24 h followed by serum starvation for 12 h. Cells were stimulated without (lanes 1 and 3) or with (lanes 2 and 4) 10 nM EGF for 3 min and lysed. Equal amounts of total proteins were immunoprecipitated with antiphosphotyrosine antibodies, and immunoprecipitates were immunoblotted with anti-EGFR antibodies (IP: pY, WB: EGFR). Lysates were immunoblotted for P-MAPK, MAPK, EGFR [short exposure (SE) and long exposure (LE)], and Pnck.
To begin to explore the mechanism underlying the restoration of EGFR levels at high cell density in HA-Pnck cells, we tested how the phenomenon is related to EGF signaling. Duplicate dishes of both Neo and HA-Pnck cells were plated at three increasing cell densities, serum starved, and then stimulated without or with EGF. Lysates were analyzed for activated MAP kinase, EGFR, HA-Pnck, and MAP kinase levels. As observed before, the lowest levels of EGFR, in the absence of ligand, were seen at the lowest densities of HA-Pnck cells, and levels gradually increased with cell density (Fig. 4B, WB: EGFR, lanes 7, 9, and 11). Again, EGFR levels did not remarkably increase with density in the Neo cells in the absence of ligand (Fig. 4B, WB: EGFR, lanes 1, 3, and 5). As before, HA-Pnck levels also increased with cell density (Fig. 4B, WB: HA-Pnck, lanes 7–12). However, despite the increased levels of EGFR in HA-Pnck cells at high density in the absence of EGF, when the cells were treated with the ligand, EGF-induced MAP kinase activation was paradoxically found to be lowest in HA-Pnck cells at the highest cell density (Fig. 4B, WB: P-MAPK lanes 8, 10, and 12). We considered the possibility that this might be due to the cell density-induced EGFR being unable to signal appropriately due, for example, to sequestration away from the plasma membrane; however, this seems very unlikely since these receptors appear able to bind EGF as seen by the efficient, ligand-induced degradation produced by treatment with EGF in the HA-Pnck cells (Fig. 4B, WB: EGFR, lanes 8, 10, and 12). A transient (3 min) stimulation of these HA-Pnck cells at the highest density with EGF also showed strong EGF-induced tyrosine phosphorylation of EGFR equivalent to that in the Neo cells (Fig. 4C, IP: pY, WB: EGFR, lanes 2 and 4). We therefore suspect that EGF-induced MAP kinase signaling is suppressed at very high cell density due to modulation of some other component of the MAP kinase signaling pathway downstream of EGFR, the identity of which is currently not known.
Pnck-induced EGFR degradation is not dependent on Pnck kinase activity.
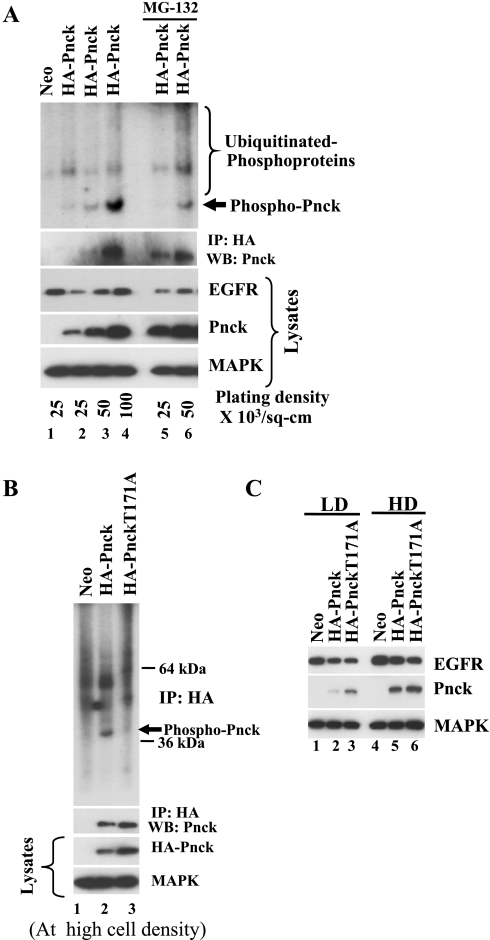
In our previous report we observed Pnck kinase activity in cells in which EGFR was undergoing ligand-independent EGFR degradation (6). Since Pnck also undergoes proteasomal degradation concomitant with ligand-independent EGFR degradation (6), we hypothesized that Pnck kinase activity is required for EGFR degradation and so set out to evaluate the role of Pnck kinase activity in the induction of EGFR degradation, in the context of different cell densities. Cells were plated at several densities and lysates were prepared for analysis in immunoblot and immunoprecipitation kinase assays as previously described (6). As in the previous experiments, immunoblotting of the lysates revealed that EGFR levels were suppressed in Pnck-expressing cells plated at low cell densities due to Pnck-induced degradation, whereas at higher cell densities, EGFR degradation was suppressed (Fig. 5A, WB: EGFR, lanes 2–4). HA-Pnck protein levels, as expected, were lowest in the cells at low cell density (WB: Pnck, lane 2) and higher in cells at high density (WB: Pnck, lanes 3 and 4). It proved to be difficult to immunoprecipitate sufficient Pnck for easy detection by Western blot analysis from the cells plated at low density (IP: HA, WB: Pnck, lanes 2 and 3); however, when the precipitates were evaluated in the kinase assay, detectable kinase activity was observed at the size expected for Pnck (Fig. 5A, top, phospho-Pnck, lanes 2 and 3). At high cell densities, however, Pnck was readily immunoprecipitated from the cell extracts (IP: HA, WB: Pnck, lane 4) and strong kinase activity was detected in the corresponding immunoprecipitate (Fig. 5A, top, phospho-Pnck, lane 4). Pnck undergoes proteasomal degradation at low cell densities, and pretreatment of the cells with the specific proteasomal inhibitor MG-132 (WB: Pnck, lanes 5 and 6) resulted in the immunoprecipitation of more Pnck (IP: HA, WB: Pnck, lanes 5 and 6) and in correspondingly higher Pnck kinase activity (Fig. 5A, top, phospho-Pnck, lanes 3 and 6) with a smear of higher-molecular-weight phosphoproteins, characteristic of ubiquitinated proteins destined for proteasomal degradation. These data suggest that kinase-active Pnck is simultaneously degraded by the proteasome concurrent with the protea-lysosomal degradation of EGFR.
Fig. 5.
Pnck-induced EGFR degradation is not dependent on Pnck kinase activity. A: wild-type (WT)-Pnck is degraded by the proteasome concurrent with EGFR degradation. Low-density Neo cells (lane 1) and HA-Pnck HEK-293 cells plated with increasing cell density (lanes 2–4) were allowed to grow for 48 h and were then lysed. Equal amounts of total protein were assayed for protein levels by immunoblotting [WB: EGFR, WB: Pnck, WB: MAPK (loading control)] and were immunoprecipitated with anti-HA mAb. Half of the immunoprecipitated materials were immunoblotted for Pnck (IP: HA, WB: Pnck) and the remaining half was subjected to immunokinase assay. Kinase reaction products were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membrane, and subjected to autoradiography. Two separate plates of low-density HA-Pnck cells were preincubated with 10 μM proteasomal inhibitor, MG-132, overnight and subjected to immunoprecipitation and kinase reactions using similar methods. A representative result from two independent experiments is presented here. B: Pnck T171A mutant exhibits reduced kinase activity. Neo, HA-Pnck, and HA-Pnck T171A HEK-293 cells were plated at high cell density, and lysates were prepared to evaluate protein levels by immunoblotting (lysates-WB: HA-Pnck and MAPK) and for immunoprecipitation. Half of the immunoprecipitated materials were subjected to immunokinase assay (top) and the remaining half was immunoblotted for Pnck (IP: HA, WB: Pnck) as described above. A representative example of two experiments is presented. C: Pnck-induced EGFR degradation is not dependent on Pnck kinase activity. Neo, HA-Pnck, and HA-Pnck T171A HEK-293 cells were plated at low (LD) and high (HD) cell density, and lysates were prepared after 48 h. Equal amounts of total protein were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: Pnck) and MAPK (loading control) expression.
As mentioned above, Pnck has limited sequence similarity to calmodulin kinase I, which is restricted to the catalytic domains of the enzymes (13). Haribabu et al. (15) demonstrated that mutation of Thr177 to alanine in human calmodulin kinase I blocked both the phosphorylation and the specific kinase activity of the enzyme (15). Furthermore, activation loop phosphorylation at Thr177 was previously shown to strongly determine the peptide substrate specificity of calmodulin kinase I (16). Through homology alignment of the human calmodulin kinase I and human Pnck primary sequences, we tentatively identified amino acid Thr171 in Pnck as being equivalent to Thr177 in calmodulin kinase I. We used site-directed mutagenesis to convert Thr171 to alanine with the goal of examining the role of Pnck kinase activity in the induction of EGFR degradation. HEK-293 cell clones stably transfected with the T171A mutant were generated, and initial experiments were conducted to verify that the T171A mutant has impaired kinase activity. Lysates were prepared from Neo, HA-Pnck, and HA-Pnck-T171A cells grown at high density for immunoblot and IP-kinase assay as before. Although both HA-Pnck and the T171A mutant were readily immunoprecipitated from the lysates, significant kinase activity was only detected in the HA-Pnck sample, even though the levels of the mutant protein were, if anything, higher in both the lysates and the immunoprecipitates (Fig. 5B). To assess the ability of the T171A mutant to induce the degradation of EGFR, lysates were prepared from the Neo control cells, from cells expressing the mutant, and from the WT HA-Pnck cells grown both at low and high cell densities to evaluate EGFR and Pnck levels (Fig. 5C). As before, expression of WT HA-Pnck at low cell density resulted in substantial suppression of EGFR levels (Fig. 5C, WB: EGFR, lane 2). Interestingly, EGFR levels were suppressed to essentially the same extent in the cells expressing the T171A mutant Pnck, suggesting that Pnck-induced EGFR degradation is not regulated by Pnck kinase activity (Fig. 5C, WB: EGFR, lane 3). Again, at high cell densities, there was little difference between the effect of WT and T171A mutant Pnck constructs on EGFR levels (Fig. 5C, WB: EGFR, lane 6).
Posttranslational modification of Hsp90 (mass spectrometric analysis of Hsp90).
On the basis of the finding that Pnck is an Hsp90 client (Fig. 2), the intriguing observation that the electrophoretic mobility of Hsp90 is slightly reduced in Pnck-overexpressing HEK-293 cells (Fig. 4), the finding that Pnck kinase activity is not required for ligand-independent EGFR degradation (Fig. 5), and the previous reports that Hsp90 chaperone activity can be modulated by posttranslational modifications including phosphorylation (55), we wondered whether Hsp90 might be posttranslationally modified directly or indirectly by Pnck. To examine this possibility, we immunoprecipitated Hsp90 from both the Neo, HA-Pnck, and T171A Pnck HEK-293 cells and subjected the material to proteomic analysis. Immunoprecipitated materials were resolved on an SDS-PAGE gel and stained with Coomassie Blue G-250. The bands corresponding to a mass of 90 kDa were excised and subjected to in-gel proteolytic digestion, and peptide analysis by nano-LC-MS/MS. Samples from three independent immunoprecipitation experiments were subjected to three rounds of analysis with full scan ion chromatograms recorded in an information-dependent acquisition (IDA) mode to acquire the MS/MS data. The IDA data were then analyzed employing Protein Pilot 3.0 (Applied Biosystems) database search software using the Swiss Prot database. Hsp90-α and -β were identified with 99% confidence with a sequence coverage of 63%, with 25 associated peptides identified at a confidence of >95%. The MS/MS spectra were manually validated for the presence of the modification identified by database search. The full scan chromatograms were analyzed to assign the charge state and retention times. The fragmentation spectra of the phosphorylated peptides are shown in Supplemental Fig. S2 along with a more detailed explanation of the analysis. On the basis of this analysis, the results of which are summarized in Table 1, we established that both HSP90-α (accession no. P07900) and Hsp90-β (accession no. P08238) were phosphorylated at threonines 616 and 89 (Supplemental Fig. S2, A and B) and Hsp90-α at serine 391 in extracts from HA-Pnck cells (Supplemental Fig. S2C), and that these modifications were not present in extracts from the Neo control cells. Consistent with the findings with the Neo control cells, modification at these residues was also not detected on analysis of Hsp90 immunoprecipitates from the T171A cells (Table 1).
Table 1.
LC-ESI-MS profile of the phosphorylated tryptic peptides of Hsp90
| Residue | Sequence | Pnck WT (Modified) [m/z (z)], M+, RT | Pnck T171A (Nonphosphorylated) [m/z (z)], M+, RT | Neo (Nonphosphorylated) [m/z (z)], M+, RT |
|---|---|---|---|---|
| 613–623 | DNST*MGYMMAK | [656.74(2)], 1,311.47, 16.5 | [640.75(2)], 1,279.48, 16.2 | [648.74(2)], 1,295.47, 16.1 |
| 83–95 | TLTLVDT*GIGMTK | [691.36(2)], 1,380.72, 24.0 | [683.37(3)], 1,364.72, 22.5 | [683.36(2)], 1,364.7, 22.4 |
| 388–400 | VVDS*EDLPLNISR | [522.90(3)], 1,565.69, 23.7 | [757.39(2)], 1,512.78, 23.4 | [757.39(2)], 1,512.77, 23.3 |
Tryptic digests of heat shock protein 90 (Hsp90) protein were subjected to nanoLC-ESI/MS/MS. Two independent full scan ion chromatograms were recorded in an information-dependent acquisition (IDA) mode to acquire MS/MS data. The IDA data were then analyzed with the Protein Pilot database search software (ABSCIEX) using the Uniprot database. The MS/MS data were analyzed manually to confirm the sequences of the modified and the unmodified forms of the same peptide identified by the database search. The full scan chromatograms were analyzed to assign the charged state and the retention times (RT; in min). The peptides were phosphorylated in the pregnancy-upregulated nonubiquitous calmodulin kinase-wild type (Pnck WT) sample and nonphosphorylated in the Neo and the mutant samples. The 79-Da mass difference between the modified and the unmodified peptides appeared because of the phosphorylation of the serine or threonine residue. The peptide VVDS * EDLPLNISR was found in the Pnck WT sample. The corresponding peptide in the Neo and mutant samples was longer by one amino acid (G) at the NH2 terminus. M+, precursor molecular weight.
Pnck promotes cellular proliferation in HEK-293 cells despite inducing EGFR degradation.
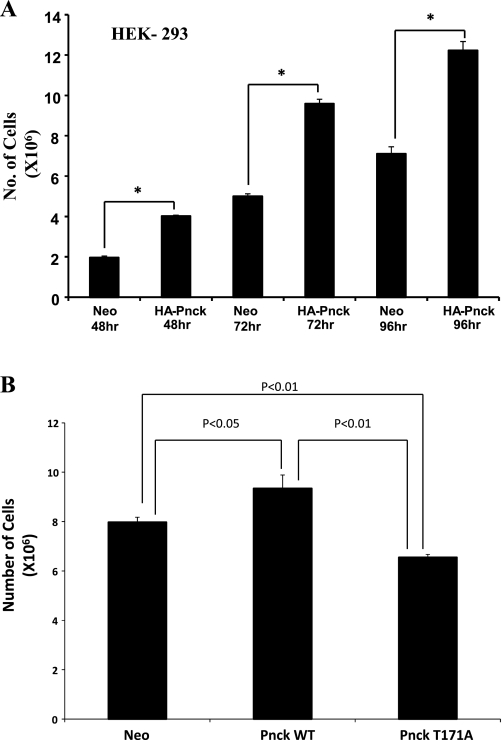
From the outset, we noticed that the WT Pnck-expressing cells consistently grew more rapidly than the Neo cells despite the suppressed EGFR levels observed in the Pnck cells. To document this observation, we plated equal numbers of both Neo and Pnck cells and monitored their proliferation by counting the cells after 48, 72, and 96 h. As observed in Fig. 6A, WT Pnck-expressing cells proliferated at a significantly higher rate, resulting in elevated cell numbers at all the time periods examined. In a separate experiment, we examined the relative proliferation of mutant (T171A) cells compared with both the Neo and WT Pnck cells by examining the increase in cell numbers over 96 h. As observed in Fig. 6B, the T171A mutant cells proliferated at a lower rate than the WT Pnck cells, indicating that phosphorylation at Thr171 of Pnck is required for the induction of higher cellular proliferation. Regulation of cellular proliferation is probably independent of the regulation of EGFR degradation by Pnck, since EGFR downregulation was observed in both WT and mutant cells (Fig. 5C).
Fig. 6.
Pnck induces cellular proliferation in HEK 293 cells. A: enhanced proliferation of Pnck-overexpressing HEK-293 cells. Nine 60-mm dishes were plated with equal numbers of Neo and HA-Pnck cells in complete medium, and a set of triplicate dishes of each cell lines were trypsinized at 48, 72, and 96 h and counted by hemocytometer. A commercial statistical software package (GraphPad Prism, version 4.03) was used to analyze data, which are expressed as means ± SD of triplicate samples. One-way analysis of variance followed by Turkey's multiple-comparisons tests were used to determine the statistical significance of differences between groups. *P < 0.001, statistically significant difference between the compared groups connected with solid lines. B: mutant (T171A) Pnck is unable to induce cellular proliferation. Neo, HA-Pnck, and HA-Pnck-T171A HEK-293 cells were plated (0.5 × 106 per dish) in triplicate in 10% heat-inactivated serum containing DMEM and counted after 96 h by hemocytometer. A representative example of three experiments is presented. A P < 0.05 value was considered significant.
WT but not mutant (T171A) Pnck promotes transition from S to G2 phase of the cell cycle.
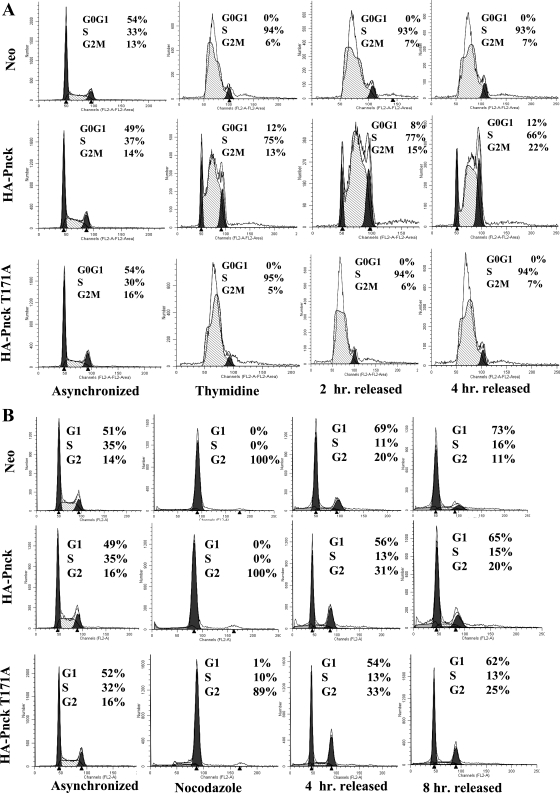
To examine alterations in the regulation of the cell cycle (if any) that might explain the enhanced growth of WT Pnck-expressing cells, we studied cell cycle progression in all three cell lines (Neo, WT, and mutant T171A Pnck) using thymidine and nocodazole in attempts to induce cell cycle arrest at the S and G2 phases, respectively, and following their behavior after release from these blocks. As observed in Fig. 7A, while the majority of Neo cells were synchronized at the S phase (94%), far fewer WT Pnck cells (only 75%) were successfully synchronized. Conversely, the T171A mutant Pnck-expressing cells behaved essentially like the Neo cells (95% synchronization), suggesting that WT Pnck promotes S to G2 phase transition. We monitored the cells up to 4 h following their release to complete medium. Both the Neo and T171A mutant cells remained synchronized up to 4 h and did not progress; however, the WT Pnck cells continuously cycled from the S to G2 to G0/G1 phases of cell cycle. In a similar fashion, both Neo and WT Pnck cells were completely synchronized at the G2 phase (100%) by nocodazole treatment, while only 89% of the mutant T171A Pnck cells accumulated in the G2 phase, leaving 10% cells at the S phase, suggesting that the T171A mutant cells have higher residency time in the S phase than the WT Pnck cells (Fig. 7B). When all three cell lines were released from the nocodazole, the WT Pnck cells progressed more rapidly than the Neo cells and at 4 h after release, the G2 fraction of WT Pnck cells (31%) was significantly higher than the Neo cells (20%) while the S phase fraction remained roughly the same (11% in Neo and 13% in WT Pnck cells), again implying a more rapid S to G2 transition induced by WT Pnck (Fig. 7B).
Fig. 7.
Pnck promotes transition from the S to G2 phase of cell cycle. A: release from thymidine synchronization. Cells were plated at low density in complete medium and allowed to recover for 48 h. Cells were then synchronized by treatment with 5 mM thymidine for 48 h, after which they were washed twice in complete medium and released in complete medium for the indicated times. Cells were trypsinized, fixed in 75% ethanol, DNA stained with propidium iodide, and analyzed by flow cytometry. B: release from nocodazole synchronization. Cells were processed as in A above, except that synchronization was performed by treatment with 50 ng/ml nocodazole for 16 h.
Cyclin-dependent kinase inhibitor Cip-1/p21/Waf-1 expression is transcriptionally downregulated by WT Pnck in a MAP kinase-dependent way in HEK-293 cells.
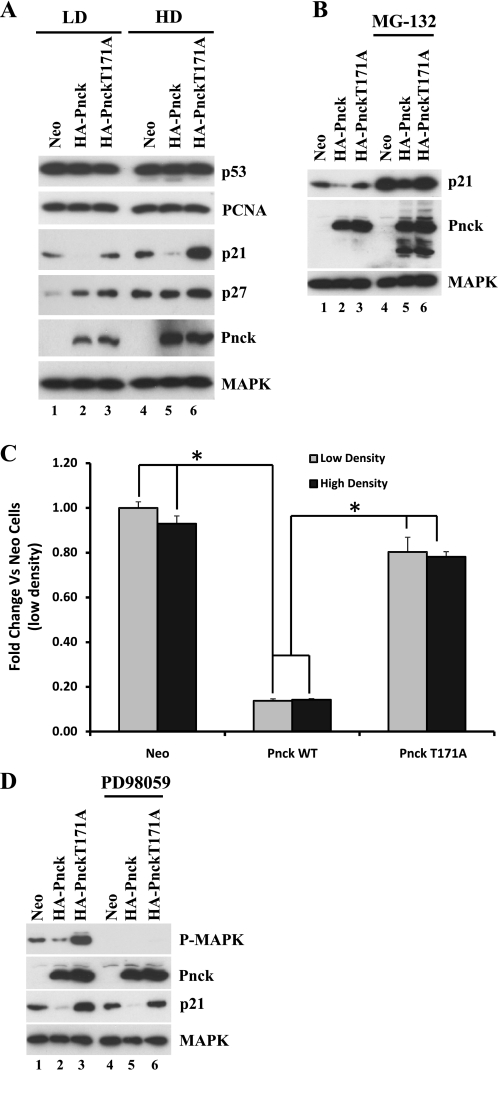
On the basis of these results, we were interested in examining the modulation of cell cycle regulators(s) by Pnck that might be responsible for this effect. The cyclin-dependent kinase (Cdk) inhibitor p21/Cip-1/Waf-1 was previously implicated in modulating cellular behavior in HEK-293 cells (10, 21, 48), so we immunoblotted lysates from Neo, WT, and mutant (T171A) Pnck-expressing cells for p21 expression. As shown in Fig. 8A, p21 expression was dramatically downregulated in the WT but not mutant Pnck-expressing cells, both at low (LD) and high (HD) cell density (WB: p21, LD and HD, lanes 1–6). This effect of Pnck seems to be relatively specific for p21 because another Cip family member, p27, was largely unchanged in WT versus mutant Pnck cells at both cell densities (Fig. 8A, WB: p27, lanes 1–6). Although p21 is not a direct Hsp90 client, previous studies have shown formation of a trimeric complex of p21/Wisp39/Hsp90, which governs posttranslational stability of p21 (20). Destabilization of this complex by geldanamycin led to proteasomal degradation of p21. Pretreatment of the cells with the proteasomal inhibitor MG-132 increased the p21 levels in all three cell lines, but the ratio of p21 levels among all cell lines remained the same in both the MG-132-treated and control cells, indicating that the effect of Pnck on p21 levels is not the result of altered proteasomal degradation (Fig. 8B, WB: p21, lanes 1–6). Ubiquitination of Pnck was observed following MG-132 treatment (as evidenced by the appearance of higher-molecular-weight forms), indicating that it undergoes proteasomal degradation as previously observed (Fig. 8B, WB: Pnck, lanes 4–6) (6). To examine whether Pnck regulates p21 mRNA expression, quantitative real-time-PCR was conducted using RNA extracted from all three cell lines grown at both low and high cell density. As seen in Fig. 8C, WT Pnck robustly reduced p21 mRNA levels by >80% compared with the Neo cells in cells at both low and high cell density (Fig. 8C). In contrast, the levels of p21 mRNA in the mutant Pnck (T171A)-expressing HEK-293 cells were not significantly different from those in HEK-293 Neo cells. It is not currently known to what extent this transcriptional downregulation of p21 by WT Pnck depends on p53, because expression of another p53 target, PCNA, was largely unaffected by Pnck expression (Fig. 8A, WB: PCNA, lanes 1–6).
Fig. 8.
Basal MAP kinase activity and cyclin-dependent kinase (Cdk) inhibitor p21/Cip-1/Waf-1 expression is inhibited by WT Pnck in HEK-293 cells. A: inhibition of p21/Cip-1/Waf-1 expression by WT Pnck but not by mutant (T171A) Pnck. Neo, HA-Pnck, and HA-Pnck T171A cells were plated either at low cell density (lanes 1–3) or at high cell density (lanes 4–6) in complete medium, allowed to grow for 48 h, and lysed. Equal amounts of total protein were immunoblotted for p53, PCNA, p21, p27, Pnck, and MAPK. B: downregulation of p21/Cip-1/Waf-1 expression by WT Pnck is not due to proteasomal degradation. Neo, HA-Pnck, and HA-Pnck T171A cells were plated and after 48 h were treated without (lanes 1–3) or with 20 μM MG-132 (lanes 4–6) for 8 h. Cells were lysed and equal amounts of total protein were immunoblotted for p21, Pnck, and MAPK expression. C: p21/Cip-1/Waf-1 is transcriptionally downregulated by WT but not by T171A Pnck. Neo, WT, and HA-Pnck T171A stable HEK-293 cells were seeded at low density or high density and cultured for 2 days. Expression of p21 mRNA was measured using quantitative real-time PCR and compared with low-density Neo cells. *P < 0.05 compared with Neo or mutated Pnck (T171A) cells. D: downregulation of basal MAP kinase activity by WT Pnck and its effect on p21/Cip-1/Waf-1 downregulation. Two sets of an equal number of Neo, WT, and HA-Pnck T171A stable HEK-293 cells were plated in complete medium. After 48 h, each set was treated without (lanes 1–3), or with 10 μM PD98059 (lanes 4–6) for 8 h. Cell lysates were immunoblotted for P-MAPK, Pnck, p21, and MAPK. Experiments were repeated three times and a representative experiment is presented.
On the basis of the previous observation of potential regulation of p21 expression by MAP kinase activity in HEK-293 cells (48) and our observation of Pnck regulation of p21 transcription, we tested Pnck's effect on basal MAP kinase activity in cells growing in serum at both low and high cell densities. Although acute treatment with serum in serum-starved low-density Neo and HA-Pnck cells did not induce significant MAP kinase activation (Fig. 1, A and B), Neo cells grown continuously in serum had detectable MAP kinase activity and this basal MAP kinase activation was downregulated in WT but not in mutant (T171A) Pnck-expressing cells (WB: P-MAPK, lanes 1–3). In fact, MAP kinase activity was much more pronounced in mutant T171A cells than even the Neo cells. As previously observed (Fig. 8, A and B), p21 expression was again downregulated in WT Pnck cells and not in the mutant cells (Fig. 8D); in fact, p21 expression was even higher than in the Neo cells, in parallel with the robust MAP kinase activity in the mutant cells (Fig. 8D, WB: P-MAPK, WB: p21, lane 3). Activation of MAP kinase is generally associated with cellular proliferation; however, depending on cellular context, intensity, and duration of MAP kinase activity, other biological outputs, such as cell cycle arrest and differentiation, have previously been observed, and in most cases activated MAP kinase induces such paradoxical phenotypes by inducing p21 protein expression (5, 47, 56, 65). Our observations of strong MAP kinase activity, increased p21 expression, growth inhibition, and accumulation in the S phase of mutant cells (T171A) but not WT Pnck cells convinced us that the MAP kinase activation was likely responsible for p21 upregulation in mutant cells, and conversely the MAP kinase inactivation/p21 downregulation in WT Pnck-expressing cells. To test this supposition, all cell lines were pretreated with a specific MEK inhibitor, PD98059, and p21 expression was monitored. As seen in Fig. 8D, p21 expression was inhibited in (T171A) mutant Pnck cells (lanes 3 and 6) coincident with MAP kinase inhibition (WB: P-MAPK, lanes 3 and 6), indicating that WT Pnck may be transcriptionally downregulating p21 expression by inhibiting MAP kinase activation.
DISCUSSION
In this communication we have established what is the likely mechanism underlying the ligand-independent degradation of EGFR induced by Pnck, a novel calcium/calmodulin kinase (Supplemental Fig. S3). This work is an extension of our previous study, in which we showed that ligand-independent EGFR degradation occurred without a requirement for EGFR tyrosine kinase activity (6), a prime requirement in c-Cbl E3-ubiquitin ligase-mediated ligand-dependent EGFR degradation (1, 9, 11, 14). We demonstrated that EGFR downregulation in Pnck-overexpressing HEK-293 cells is due to the protea-lysosomal degradation of EGFR (6). Here we present evidence that suggests that Pnck-induced EGFR degradation probably involves perturbation of the function of the ATP-dependent chaperone heat shock protein 90 (Hsp90) complex. Although EGFR biology has been studied extensively, exactly how EGFR is chaperoned by Hsp90 is not clearly understood. It has been shown that mutant EGFR requires Hsp90 protein for chaperoning (25, 57), and that Hsp90 activity is required during the synthesis of WT EGFR; however, no studies have clearly demonstrated the formation of complexes between Hsp90 and mature WT EGFR. We have been able to partially dissect the process of ligand-independent EGFR degradation through our studies of the novel kinase, Pnck. Pnck, classified as a calcium/calmodulin kinase, by virtue of the presumptive calmodulin-binding domain identified in its primary sequence (13), is distantly related to calmodulin kinase I (13); however, the homology is limited and is restricted to the catalytic domains of the proteins. Pnck mRNA is upregulated in a subset of epithelial cells in the normal mouse mammary gland during late pregnancy and is associated with these cells exhibiting reduced proliferation and undergoing terminal differentiation (12). Pnck is also overexpressed in ∼30% of human breast cancer tissue relative to adjoining benign tissues (12). However, the function of Pnck in either normal mammary gland or breast cancer is still unknown.
We demonstrated here that Pnck is an Hsp90 client protein that is associated with Hsp90 and which is degraded by treatment of cells with the pharmacologic Hsp90 inhibitor, geldanamycin. The association of Pnck with Hsp90 appears to depend on culture conditions and is strong in cells growing in serum but is largely lost when those cells are placed in serum-free conditions. It is not currently known what causes Hsp90 to associate with Pnck in serum-fed cells; an interesting possibility relates to the abundance of ATP in serum-fed cells that helps clients associate with Hsp90, since it has previously shown lack of ATP results in clients (such as ErbB2) dissociating from Hsp90 and subsequent degradation (45). Treatment with geldanamycin enhances EGFR degradation in Pnck-expressing cells. Although Pnck is categorized as a calcium/calmodulin-dependent kinase, on the basis of its homology to other proteins, in our experiments using a specific calmodulin antagonist and a calcium chelator, the effects of Pnck on EGFR levels appear to be calcium/calmodulin independent, at least in HEK-293 cells. We have previously used both of these cell-permeable inhibitors and have demonstrated in similar systems that treatment with the calmodulin inhibitor W-7 produced essentially identical biochemical endpoints to those produced by the direct suppression of calmodulin activity using siRNA-mediated knockdown of the three calmodulin genes (CALM1, 2, 3) (3, 7). Subsequent Pnck mutagenesis and immunokinase assays revealed that Pnck possesses kinase activity, albeit at low levels and low cell density, the conditions under which EGFR degradation takes place. The ability of Pnck to induce EGFR degradation does not, however, appear to depend on the level of Pnck kinase activity since a mutated Pnck protein (T171A) failed to restore EGFR levels to those seen in Neo cells. Although the Thr171 mutation does not make Pnck a kinase-dead protein (as mutation of the ATP acceptor lysine would do), mutation of this activation loop threonine strongly suppressed Pnck kinase activity in an immunokinase assay. Calmodulin kinases are classically thought to be regulated by calcium/calmodulin for full activation but are known to have kinase activity independent of calcium/calmodulin, resulting in autophosphorylation (19). In fact, Thr286 and Thr196 phosphorylation, in calmodulin kinase II and IV, respectively, generated an autonomous kinase activity which could be further activated by calcium/calmodulin (4, 61). Pnck-induced EGFR degradation is dependent on cell density, and maximal EGFR degradation was observed at low cell density, with degradation gradually being suppressed with increasing cell density. This inhibition of EGFR degradation with increasing cell density was paralleled with increased levels of HA-Pnck protein. Previously, we observed the proteasomal degradation of Pnck in cells in which EGFR is degraded (6); thus, increased Pnck levels at high cell density could result from the inhibition of proteasomal activity. From the kinase studies we determined that at low cell density, kinase-active Pnck was undergoing degradation in parallel with EGFR and that Pnck destruction was reversed by pretreatment of the cells with the proteasomal inhibitor MG-132, resulting in the accumulation of ubiquitinated phosphoproteins (6). The apparent instability of Pnck at low cell density could be related to its low level of kinase activity, since higher levels of kinase activity are responsible for conferring Pnck stability. At high cell density, under conditions of high levels of WT Pnck protein and with significant kinase activity present, Pnck did not induce EGFR degradation, again indicating a disconnect between Pnck kinase activity and EGFR degradation. Throughout our studies, we have considered the expression of mature EGFR protein which can subsequently respond to EGF stimulation, and the potential role of Pnck kinase activity on nascent EGFR stability as well as on the stability of other Hsp90 clients remains to be investigated.
The physical association of Pnck with Hsp90 due to its status as a novel client, and previous reports of Hsp90 posttranslational modification, including phosphorylation, that alter its chaperone ability (55), prompted us to explore the possibility that Pnck regulates the posttranslational modification of Hsp90. An initial clue was obtained from experiments in which a lower electrophoretic mobility was observed for Hsp90 in HA-Pnck cells. Pharmacologic Hsp90 inhibitors regulate client dissociation and degradation predominantly by inhibiting the Hsp90 ATPase activity, known to be required for proper chaperoning of clients by Hsp90. Besides pharmacologic inhibition, ATPase and chaperone activity have previously been shown to be regulated by four types of Hsp90 posttranslational modification, namely, phosphorylation (35, 41, 63, 71), acetylation (23, 54), S-nitrosylation (32), and ubiquitination (24, 36). Immunoblotting experiments with Hsp90 immunoprecipitated from Pnck cells using specific antibodies seemed to rule out the possibility that Pnck modulates Hsp90 posttranslational modification by acetylation and S-nitrosylation in HEK-293 cells (data not shown), and so we decided to take a more agnostic approach to identify possible Hsp90 posttranslational modifications caused by Pnck. Using mass spectrometric-based proteomic analysis of Hsp90 isolated from both Neo, Pnck, and mutant T171A Pnck-overexpressing cells, we were able to demonstrate strong Hsp90 phosphorylation at specific residues in Pnck cells but not in the Neo or mutant T171A cells. No other differential posttranslational modification of Hsp90 caused by Pnck expression was observed. Two threonine (Thr89 and Thr616) residues in both Hsp90-α and -β and one serine residue (Ser391) in Hsp90-α were found to be strongly phosphorylated only in Pnck-overexpressing HEK-293 cells and not in Neo or mutant T171A cells. These sites are located at the NH2 terminus (Thr89), responsible for regulating ATPase activity and geldanamycin binding, in the middle domain (Ser391) and in the COOH terminus (Thr616) responsible for the dimerization (43, 44, 46) of Hsp90, suggesting the possibility of a global Hsp90 conformational change caused by Pnck.
Previous studies have strongly implicated Hsp90 phosphoregulation as an important mechanism for the regulation of Hsp90 client maturation and function. In human cells, protein phosphatase inhibition by okadaic acid increased Hsp90 phosphorylation (2-fold increase in serine and 20-fold in threonine phosphorylation), which in turn inhibited the maturation of v-Src by destabilizing the complex formation between pp60v-Src and Hsp90 resulting in a reduction in the plasma membrane pool of v-Src (35). Protein phosphatase 5 (PP5) forms complexes with Hsp90 (2, 58, 62), and Ppt1 (yeast homologue of protein phosphatase 5) was shown to be a dedicated regulator of Hsp90 (63). Yeast strains with genomic deletion of Ppt1 exhibited increased Hsp90 phosphorylation. Hsp90 phosphorylation was also linked with the maturation of reovirus attachment protein σ1 which was dissociated following Hsp90 phosphorylation (71). Functionally, Hsp90 phosphorylation was shown to inhibit ligand-induced transcriptional activity of the Aryl hydrocarbon receptor complex (AhR) (41). Interestingly, though phosphoregulation of Hsp90 is considered to be an important mechanism regulating client protein maturation, a specific Hsp90 kinase has yet to be characterized in detail. Casein kinase II and double-stranded DNA kinase were previously described as Hsp90 kinases (26, 27, 51); however, little further information is available regarding the regulation of Hsp90 by these kinases. It is worth mentioning that using a commercially available phosphospecific Hsp90 antibody (Cell Signaling Technology), we observed constitutive phosphorylation at Thr5 and Thr7, located at the extreme NH2 terminus of Hsp90 in both Neo and Pnck cells, and no Pnck-specific differential phosphorylation was observed at these amino acids (data not shown). In the current context, Pnck is a candidate kinase that might directly phosphorylate Hsp90 at the sites we have identified by MS studies and thereby regulate its activity. There are, however, several other possible mechanisms by which Pnck might modulate Hsp90 phosphorylation. It is possible that Pnck's effect is indirect and is mediated by a downstream kinase that is activated by Pnck or that is brought into close proximity by Hsp90 through interaction with Pnck. A third possibility is that Pnck inhibits a phosphatase residing within the Hsp90 complex, thereby increasing Hsp90 phosphorylation, or that it activates a phosphatase which in turn activates a proximal Hsp90 kinase. As mentioned above, the tetratricopeptide repeat (TPR) domain containing protein phosphatase Ppt1 (yeast) and its mammalian homologue PP5 are known to interact with a COOH-terminal Hsp90 TPR-binding motif (2). This phosphatase was also shown to dephosphorylate an Hsp90 cohort, cdc37, in tumor cells, leading to the downregulation of an Hsp90 client (62). The possibility that Pnck phosphorylates a co-chaperone besides Hsp90 remains to be investigated. We observed the specific phosphorylation of a protein at ∼60 kDa that coimmunoprecipitated with WT but not with mutant Pnck (Fig. 5B); however, the identity of this protein is currently unknown.
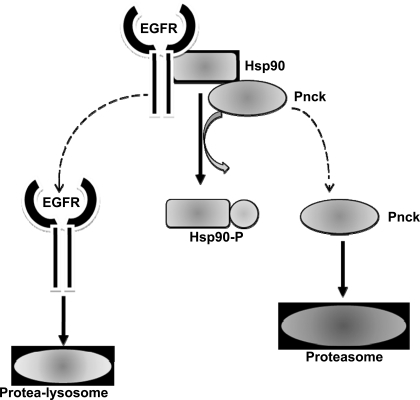
In conclusion, we have described a novel mechanism by which Pnck may be involved in the regulation of cellular chaperones and the consequences on EGFR stability. This work leads us to propose a hypothetical model whereby Pnck-induced Hsp90 phosphorylation mediates the perturbation of the chaperone ability of Hsp90 complex, resulting in the subsequent dissociation and degradation of Hsp90 clients including EGFR and Pnck (Fig. 9). Although our study clearly indicates a strong role for Pnck in modulating Hsp90 phosphorylation, several questions remain unanswered at this point. First, it is not clear whether Pnck-induced Hsp90 phosphorylation is the result of a stress response. We do not understand how Pnck is activated in a calcium/calmodulin-independent manner, or to what degree Hsp90 ATPase activity is modulated. Second, it is not known whether Pnck's effect on Hsp90 activity results in general effects on chaperone activity or whether this only impacts selective Hsp90 clients. We were readily able to detect the Pnck-induced Hsp90 phosphorylation by mass spectra analysis in immunopurified Hsp90, suggesting that this phosphorylation is present on the majority of Hsp90 molecules within the cells. If this is the case, one would expect Pnck to affect Hsp90 clients (other than EGFR) associated with different pools of Hsp90 in the cells. Since EGFR expression and degradation are ubiquitous events and Pnck is a nonubiquitous protein, it is likely that the ligand-independent EGFR degradation mechanism(s) other than those mediated by Pnck exist. Third, the biological consequences of Hsp90 phosphorylation, client degradation, and the regulation of MAP kinase activity in human breast cancer, and the true physiological role of Pnck, remain unclear. In our studies we observed strong inhibition of p21/Cip-1/Waf-1 expression in WT Pnck cells which was apparently downstream of basal MAP kinase inhibition, and conversely, p21 expression and MAP kinase activation in T171A mutant cells. Expression of this mutant also failed to increase cell proliferation and cell cycle progression from the S to G2 phase, and so behaves as a “loss of function” mutant, at least in HEK-293 cells. Our data suggest that the regulation of basal MAP kinase activity and p21 expression by Pnck occurs in an EGFR-independent manner, probably due to additional effects of Pnck on a post-EGFR component of the serum-induced MAP kinase signaling axis. This component of the serum-MAP kinase axis could be identical to the component of the EGFR-MAP kinase axis which is downregulated/inactivated at high cell density thus inducing strong suppression of EGF-induced MAP kinase activity. Identification of this component is the focus of intense investigation, and we speculate that it is robustly regulated by Pnck in either an Hsp90-dependent or -independent manner. Our observation of MAP kinase activation, p21 upregulation, and the paradoxical inhibition of cell cycle progression in mutant Pnck-expressing HEK-293 cells is consistent with previous observations of calcitonin receptor-induced arrest in HEK-293 cells, involving a similar MAP kinase/p21 signaling axis (10, 48). MAP kinase activation is classically considered a pro-proliferative event; however, previous observations have indicated that several factors, such as cellular context (normal or transformed) and the intensity and duration of MAP kinase activation, can modulate the biological outcome resulting from MAP kinase activity. Robust activation of Ras or Raf, the upstream proteins in the MAP kinase signaling axis, has been shown to induce cell cycle arrest rather than proliferation in certain contexts, and, furthermore, this arrest is associated with upregulation of p21 expression (47, 56, 65). On the basis of our observation of prominent regulation of both basal and ligand-induced MAP kinase activity in HEK-293 cells, examination of MAP kinase regulation and biology by Pnck in transformed cells, such as in human breast cancer, is warranted.
Fig. 9.
A proposed hypothetical scheme of Pnck-induced ligand-independent EGFR degradation. Pnck-induced Hsp90 phosphorylation leads to inhibition of Hsp90 chaperone activity thus inducing EGFR dissociation from Hsp90. Dissociated EGFR undergoes protea-lysosomal degradation along with proteasomal degradation of Pnck.
GRANTS
This work was supported in part by an award from the Susan G. Komen for the Cure Foundation (BCTR0707114; to T. B. Deb), by American Cancer Society Grant IRG-97-152-16 through an individual allocation by the Lombardi Comprehensive Cancer Center (to T. B. Deb), and by National Institutes of Health Grant 2RO1-AG014963 (to M. D. Johnson). Assistance from LCCC core facilities (Tissue Culture Shared Resource, Microscopy and Imaging Shared Resource, Flow Cytometry and Cell Sorting Shared Resource, Proteomics and Metabolomics Shared Resource), which are supported by Cancer Center Support Grant 5P30CA051008-16, is gratefully acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank Dr. Lihua Zhang, Proteomics Shared Resources, Lombardi Comprehensive Cancer Center (LCCC), for processing samples for mass spectrometric analysis and Annie Park and Dr. Karen Cresswell, LCCC, for excellent cell cycle analysis by flow cytometry.
Present addresses: Y. Wang, Chemotaxis Signal Section, Laboratory of Immunogenetics, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20814; C. M. Coticchia, Vascular Biology Program and Department of Surgery, Children's Hospital Boston and Harvard Medical School, Boston, MA 02115.
REFERENCES
- 1. Bowtell DD, Langdon WY. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene 11: 1561–1567, 1995 [PubMed] [Google Scholar]
- 2. Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem 271: 32315–32320, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Coticchia CM, Revankar CM, Deb TB, Dickson RB, Johnson MD. Calmodulin modulates Akt activity in human breast cancer cell lines. Breast Cancer Res Treat 115: 545–560, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coultrap SJ, Buard I, Kulbe JR, Dell'Acqua ML, Bayer KU. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem 285: 17930–17937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77: 841–852, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Deb TB, Coticchia CM, Barndt R, Zuo H, Dickson RB, Johnson MD. Pregnancy-upregulated nonubiquitous calmodulin kinase induces ligand-independent EGFR degradation. Am J Physiol Cell Physiol 295: C365–C377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem 279: 38903–38911, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Deb TB, Wong L, Salomon DS, Zhou G, Dixon JE, Gutkind JS, Thompson SA, Johnson GR. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J Biol Chem 273: 16643–16646, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Ettenberg SA, Magnifico A, Cuello M, Nau MM, Rubinstein YR, Yarden Y, Weissman AM, Lipkowitz S. Cbl-b-dependent coordinated degradation of the epidermal growth factor receptor signaling complex. J Biol Chem 276: 27677–27684, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Evdokiou A, Raggatt LJ, Atkins GJ, Findlay DM. Calcitonin receptor-mediated growth suppression of HEK-293 cells is accompanied by induction of p21WAF1/CIP1 and G2/M arrest. Mol Endocrinol 13: 1738–1750, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Galisteo ML, Dikic I, Batzer AG, Langdon WY, Schlessinger J. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem 270: 20242–20245, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Gardner HP, Ha SI, Reynolds C, Chodosh LA. The CaM kinase, Pnck, is spatially and temporally regulated during murine mammary gland development and may identify an epithelial cell subtype involved in breast cancer. Cancer Res 60: 5571–5577, 2000 [PubMed] [Google Scholar]
- 13. Gardner HP, Rajan JV, Ha SI, Copeland NG, Gilbert DJ, Jenkins NA, Marquis ST, Chodosh LA. Cloning, characterization, and chromosomal localization of Pnck, a Ca(2+)/calmodulin-dependent protein kinase. Genomics 63: 279–288, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Grovdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res 300: 388–395, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Haribabu B, Hook SS, Selbert MA, Goldstein EG, Tomhave ED, Edelman AM, Snyderman R, Means AR. Human calcium-calmodulin dependent protein kinase I: cDNA cloning, domain structure and activation by phosphorylation at threonine-177 by calcium-calmodulin dependent protein kinase I kinase. EMBO J 14: 3679–3686, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hook SS, Kemp BE, Means AR. Peptide specificity determinants at P-7 and P-6 enhance the catalytic efficiency of Ca2+/calmodulin-dependent protein kinase I in the absence of activation loop phosphorylation. J Biol Chem 274: 20215–20222, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal 2: re6, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Hutchison KA, Czar MJ, Scherrer LC, Pratt WB. Monovalent cation selectivity for ATP-dependent association of the glucocorticoid receptor with hsp70 and hsp90. J Biol Chem 267: 14047–14053, 1992 [PubMed] [Google Scholar]
- 19. Ikeda A, Okuno S, Fujisawa H. Studies on the generation of Ca2+/calmodulin-independent activity of calmodulin-dependent protein kinase II by autophosphorylation. Autothiophosphorylation of the enzyme. J Biol Chem 266: 11582–11588, 1991 [PubMed] [Google Scholar]
- 20. Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B, Fotedar R, Fotedar A. Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding TPR protein. Mol Cell 17: 237–249, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kamei J, Toyofuku T, Hori M. Negative regulation of p21 by beta-catenin/TCF signaling: a novel mechanism by which cell adhesion molecules regulate cell proliferation. Biochem Biophys Res Commun 312: 380–387, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc Natl Acad Sci USA 103: 11318–11322, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18: 601–607, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kundrat L, Regan L. Identification of residues on Hsp70 and Hsp90 ubiquitinated by the cochaperone CHIP. J Mol Biol 395: 587–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavictoire SJ, Parolin DA, Klimowicz AC, Kelly JF, Lorimer IA. Interaction of Hsp90 with the nascent form of the mutant epidermal growth factor receptor EGFRvIII. J Biol Chem 278: 5292–5299, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Lees-Miller SP, Anderson CW. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem 264: 17275–17280, 1989 [PubMed] [Google Scholar]
- 27. Lees-Miller SP, Anderson CW. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem 264: 2431–2437, 1989 [PubMed] [Google Scholar]
- 28. Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell 4: 1029–1040, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev 12: 3663–3674, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol 6: 352–366, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys 58: 903–913, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Martinez-Ruiz A, Villanueva L, Gonzalez dO, Lopez-Ferrer D, Higueras MA, Tarin C, Rodriguez-Crespo I, Vazquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA 102: 8525–8530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCollum AK, Teneyck CJ, Sauer BM, Toft DO, Erlichman C. Up-regulation of heat shock protein 27 induces resistance to 17-allylamino-demethoxygeldanamycin through a glutathione-mediated mechanism. Cancer Res 66: 10967–10975, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem 271: 22796–22801, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Mimnaugh EG, Worland PJ, Whitesell L, Neckers LM. Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J Biol Chem 270: 28654–28659, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Morales JL, Perdew GH. Carboxyl terminus of hsc70-interacting protein (CHIP) can remodel mature aryl hydrocarbon receptor (AhR) complexes and mediate ubiquitination of both the AhR and the 90 kDa heat-shock protein (hsp90) in vitro. Biochemistry 46: 610–621, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mosesson Y, Yarden Y. Oncogenic growth factor receptors: implications for signal transduction therapy. Semin Cancer Biol 14: 262–270, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Nadeau K, Das A, Walsh CT. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J Biol Chem 268: 1479–1487, 1993 [PubMed] [Google Scholar]
- 39. Neckers L. Chaperoning oncogenes: Hsp90 as a target of geldanamycin. Handb Exp Pharmacol: 259–277, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Neckers L, Ivy SP. Heat shock protein 90. Curr Opin Oncol 15: 419–424, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Ogiso H, Kagi N, Matsumoto E, Nishimoto M, Arai R, Shirouzu M, Mimura J, Fujii-Kuriyama Y, Yokoyama S. Phosphorylation analysis of 90 kDa heat shock protein within the cytosolic arylhydrocarbon receptor complex. Biochemistry 43: 15510–15519, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Panaretou B, Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J 17: 4829–4836, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pearl LH, Prodromou C. Structure and in vivo function of Hsp90. Curr Opin Struct Biol 10: 46–51, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Pearl LH, Prodromou C. Structure and mechanism of the hsp90 molecular chaperone machinery. Annu Rev Biochem 75: 271–294, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Peng X, Guo X, Borkan SC, Bharti A, Kuramochi Y, Calderwood S, Sawyer DB. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem 280: 13148–13152, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets 3: 301–323, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Pumiglia KM, Decker SJ. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA 94: 448–452, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Raggatt LJ, Evdokiou A, Findlay DM. Sustained activation of Erk1/2 MAPK and cell growth suppression by the insert-negative, but not the insert-positive isoform of the human calcitonin receptor. J Endocrinol 167: 93–105, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Redpath NT, Foulstone EJ, Proud CG. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J 15: 2291–2297, 1996 [PMC free article] [PubMed] [Google Scholar]
- 50. Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42: 260–266, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Rose DW, Wettenhall RE, Kudlicki W, Kramer G, Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry 26: 6583–6587, 1987 [DOI] [PubMed] [Google Scholar]
- 52. Sakagami M, Morrison P, Welch WJ. Benzoquinoid ansamycins (herbimycin A and geldanamycin) interfere with the maturation of growth factor receptor tyrosine kinases. Cell Stress Chaperones 4: 19–28, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sargent ER, Gomella LG, Belldegrun A, Linehan WM, Kasid A. Epidermal growth factor receptor gene expression in normal human kidney and renal cell carcinoma. J Urol 142: 1364–1368, 1989 [DOI] [PubMed] [Google Scholar]
- 54. Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of hsp90 regulates chaperone function. Mol Cell 25: 151–159, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scroggins BT, Neckers L. Post-translational modification of heat-shock protein 90: impact on chaperone function. Exp Opin Drug Disc 2: 1403–1414, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Sewing A, Wiseman B, Lloyd AC, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol 17: 5588–5597, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res 65: 6401–6408, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor·hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem 272: 16224–16230, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Stern DF. Tyrosine kinase signalling in breast cancer: ErbB family receptor tyrosine kinases. Breast Cancer Res 2: 176–183, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Supino-Rosin L, Yoshimura A, Yarden Y, Elazar Z, Neumann D. Intracellular retention and degradation of the epidermal growth factor receptor, two distinct processes mediated by benzoquinone ansamycins. J Biol Chem 275: 21850–21855, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Tokumitsu H, Hatano N, Inuzuka H, Yokokura S, Nozaki N, Kobayashi R. Mechanism of the generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase IV. J Biol Chem 279: 40296–40302, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Vaughan CK, Mollapour M, Smith JR, Truman A, Hu B, Good VM, Panaretou B, Neckers L, Clarke PA, Workman P, Piper PW, Prodromou C, Pearl LH. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell 31: 886–895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wandinger SK, Suhre MH, Wegele H, Buchner J. The phosphatase Ppt1 is a dedicated regulator of the molecular chaperone Hsp90. EMBO J 25: 367–376, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 5: 761–772, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol 17: 5598–5611, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, Neckers L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem 276: 3702–3708, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Xu W, Mimnaugh EG, Kim JS, Trepel JB, Neckers LM. Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones 7: 91–96, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu W, Yuan X, Xiang Z, Mimnaugh E, Marcu M, Neckers L. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat Struct Mol Biol 12: 120–126, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Yano A, Tsutsumi S, Soga S, Lee MJ, Trepel J, Osada H, Neckers L. Inhibition of Hsp90 activates osteoclast c-Src signaling and promotes growth of prostate carcinoma cells in bone. Proc Natl Acad Sci USA 105: 15541–15546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Zhao YG, Gilmore R, Leone G, Coffey MC, Weber B, Lee PW. Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the reovirus cell attachment protein. J Biol Chem 276: 32822–32827, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.