Fig. 6.
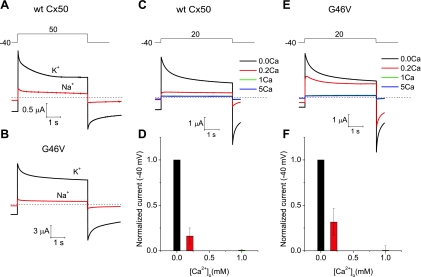
Wild-type and mutant Cx50 hemichannel currents are regulated by monovalent and divalent cations. A and B: membrane currents recorded in oocytes expressing wild-type Cx50 (A) and G46V (B) during repetitive voltage-clamp steps to 50 mV from a holding potential of −40 mV. The oocytes were initially bathed in 89 mM KCl containing zero added extracellular Ca2+ concentration ([Ca2+]o; black trace). Equimolar replacement of K+ with Na+ in the continued presence of zero added [Ca2+]o caused a large reduction in the amplitude of the hemichannel current (red trace). C: example of membrane currents recorded from a wild-type Cx50-expressing oocyte at different [Ca2+]o. The oocyte was sequentially exposed to 89 mM KCl containing Ca2+ concentrations of 0, 0.2, 1, and 5 mM. D: bar graph summarizing the concentration-response data for Ca2+ inhibition of wild-type Cx50 hemichannel currents in external K+. The current amplitudes at each Ca2+ concentration were normalized to the responses obtained in 0 mM [Ca2+]o. Data are presented as means ± SE (n = 7). E: example of membrane currents recorded from a G46V-expressing oocyte at different [Ca2+]o. The oocyte was sequentially exposed to 89 mM KCl containing Ca2+ concentrations of 0, 0.2, 1, and 5 mM. F: bar graph summarizing the concentration-response data for Ca2+ inhibition of G46V hemichannel currents in external K+. The current amplitudes at each Ca2+ concentration were normalized to the responses obtained in 0 mM [Ca2+]o. Data are means ± SE (n = 6). To reduce the size of the G46V hemichannel currents to levels comparable to those observed in wild-type Cx50-expressing oocytes, we injected oocytes with ∼800-fold lower amounts of G46V than wild-type Cx50 cRNA.