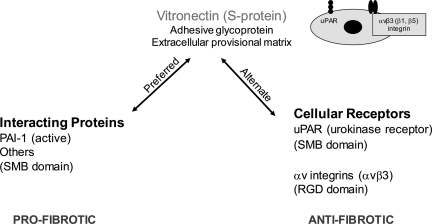
Fig. 12.
Schematic overview of the competing fibrogenic effects of Vtn. Vtn binds with high affinity to PAI-1 via its NH2-terminal somatomedin B domain (SMB), an interaction that is preferred over Vtn receptor engagement, and results in PAI-1 stability in its active conformation. PAI-1 is a potent renal fibrosis-promoting molecule, mediated at least in part by its ability to enhance interstitial myofibroblast recruitment. Although less extensively investigated, Vtn has been reported to bind an array of other molecules implicated in chronic kidney disease, including transforming growth factor-β, hepatocyte growth factor, connective tissue growth factor, epidermal growth factor, vascular endothelial growth factor, fibroblast growth factor, insulin-like growth factor II, anti-thrombin complexes, and proteoglycans. Alternatively, Vtn can interact directly with cell membrane receptors, most notably the classical uPAR via the SMB domain and αv integrins (mainly αvβ3) via its RGD domain. Both uPAR and αvβ3 have anti-fibrotic effects based on in vivo studies comparing outcomes between wild-type and receptor-deficient or -inhibited mice.