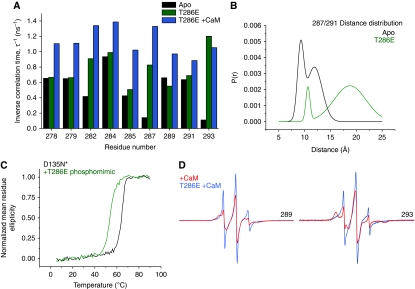
Figure 4.
(A) Comparison of the inverse rotational correlation time of spin labels in three CaMKII intermediates showing the increase in dynamics of the R1 and R2 segments in the presence of Ca2+/CaM and in the T286E mutant. (B) The phosphorylation-mimicking mutation T286E destabilizes the R1 helix leading to an increase in the width of the distance distribution between (i, i+4) spin label pairs. (C) R1 helix unfolding is manifested by a shift in the melting temperature of T286E. (D) Representative EPR lineshapes highlighting the increased dynamics of the regulatory domain in the CaM-bound T286E mutant.