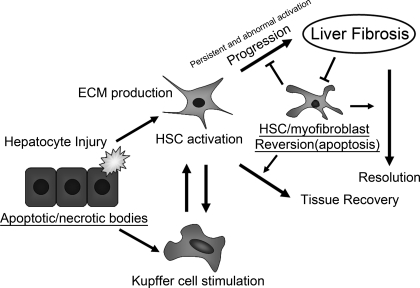
Fig. 1.
A proposed model showing how apoptosis in different cell types interacts with the inflammatory process and the progression of liver fibrosis. Proapoptotic stimuli and/or hepatotoxic reagents induce hepatocyte apoptosis/necrosis. Whereas proapoptotic signals (e.g., Fas/CD95, tumor necrosis factor-α, cathepsin B, CCAAT/enhancer-binding protein homologous protein, reactive oxygen species, etc.) enhance hepatocyte damage, antiapoptotic signals (e.g., Bcl-xL) attenuate its damage. These apoptotic/necrotic cells stimulate hepatic stellar cells (HSCs) and Kupffer cells. HSCs undergo activation, transdifferentiate into myofibroblast-like cells, and produce extracellular matrix (ECM) for reconstruction of damaged tissues. Stimulated Kupffer cells and activated HSCs produce profibrogenic cytokines (e.g., transforming growth factor-β) that, in turn, upregulate ECM production in activated HSCs. If injury ceases, the activated HSCs revert to a quiescent state (e.g., apoptosis). If injury persists, the activated HSCs and/or myofibroblasts continuously produce ECM, and a stiffer ECM further promotes ECM production in these cells. The upregulated expression of tissue inhibitor of matrix metalloproteinase-1 inhibits matrix metalloproteinase-mediated ECM degradation and renders activated HSCs and/or myofibroblasts relatively resistant to apoptotic stimuli. Such a cycling results in the progression of liver fibrosis.