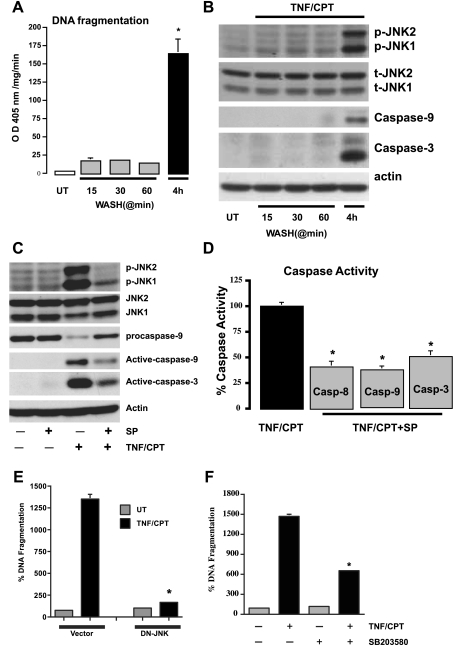
Fig. 3.
Persistent signaling by TNF + CPT is essential for the activation of JNK1/2. A: control cells left untreated or exposed to TNF + CPT for indicated time periods followed by washing with control medium were further incubated without TNF + CPT for up to 4 h. DNA fragmentation was measured to assay apoptosis. Values are means ± SE of triplicates. *Significantly different from all groups. B: cells were treated as described in A, and whole cell extracts (25 μg) were subjected to electrophoresis (SDS-PAGE) followed by Western blot analysis for detection of phosphorylated JNK1/2 and respective total JNK1/2 (t-JNK1/2) proteins using specific antibodies. Levels of active caspases-9 and -3 were determined using specific antibodies. Actin immunoblotting was performed as an internal control for equal loading. Blots are representative of 3 observations. C: cells were exposed to TNF + CPT in the presence or absence of SP-600125 (10 μM) for 4 h. Whole cell extracts (25 μg) were subjected to SDS-PAGE followed by Western blot analysis for detection of phosphorylated JNK1/2 and respective total proteins using specific antibodies. Levels of pro- and active caspases-9 and -3 were determined using specific antibodies. D: cells were exposed to TNF + CPT in the presence or absence of SP-600125 (10 μM) for 4 h. Enzymatic activities of caspases-8, -9, and -3 (Casp-8, -9, and -3) were determined from extracts prepared as described in materials and methods. Values are means ± SE of triplicates. *Significantly different from TNF + CPT. E: vector and dominant-negative (DN) JNK-transfected cells were treated with TNF + CPT for 4 h, and DNA fragmentation was measured to assay apoptosis. Values are means ± SE of triplicates. *Significantly different from TNF + CPT-treated vector cells. F: cells were exposed to TNF + CPT in the presence or absence of SB-203580 (10 μM) for 4 h, and DNA fragmentation was measured to assay apoptosis. Values are means ± SE of triplicates. *Significantly different from TNF + CPT-treated cells.