Abstract
Many of the cellular effects of glial cell line-derived neurotrophic factor are initiated by binding to GNDF family receptor alpha-1 (GFRα1), and mediated by diverse intracellular signaling pathways, most notably through the Ret tyrosine kinase. Ret may be activated by the cell autonomous expression of GFRα1 (‘in cis’), or by its non-cell autonomous presence (‘in trans’), in either a soluble or immobilized state. GFRα1 is expressed in the striatum, a target of the dopaminergic projection of the substantia nigra. To determine whether post-synaptic expression of GFRα1 in striatum in trans has effects on the development or adult responses to injury of dopamine neurons, we have created transgenic mice in which GFRα1 expression is selectively increased in striatum and other forebrain targets of the dopaminergic projection. Post-synaptic GFRα1 has profound effects on the development of dopamine neurons, resulting in a 40% increase in their adult number. This morphologic effect was associated with an augmented motor response to amphetamine. In adult mice, post-synaptic GFRα1 expression did not affect neuron survival following neurotoxic lesion, but it did increase the preservation of striatal dopaminergic innervation. We conclude that post-synaptic striatal GFRα1 expression has important effects on the biology of dopamine neurons in vivo.
Keywords: apoptosis, neurotrophic factors, Parkinson’s disease, programmed cell death, striatum, substantia nigra
Mesencephalic dopamine neurons are distinguished by the multitude of functional roles that they play in human cognition, emotion and motor control (Iversen et al. 2010). They also have great importance to the neurobiology of disease because of their selective vulnerability to neurodegeneration, most notably in Parkinson’s disease. Given the many roles that they play in human health and disease, the factors that regulate their normal development have considerable importance.
While much has been learned about specification of the dopaminergic phenotype of these neurons during embryogenesis (Smidt and Burbach 2007), much less is known about their development in the postnatal period. Like many neurons, they undergo developmental cell death (Janec and Burke 1993; Oo and Burke 1997) that is regulated by limiting quantities of endogenous neurotrophic factors and determines their final adult number. Evidence for a neurotrophic role exists for brain-derived neurotrophic factor (Alonso-Vanegas et al. 1999; Baquet et al. 2005; Oo et al. 2009). Although evidence suggests that glial cell line-derived neurotrophic factor (GDNF) may also play a role as a neurotrophic factor for these neurons (reviewed in Burke 2010), definitive gene ablation experiments have yet to be achieved, because of the perinatal lethality of conventional null mutations of GDNF (Pichel et al. 1996; Sanchez et al. 1996) and its receptor GFRα1 (GNDF family receptor alpha-1) (Cacalano et al. 1998; Enomoto et al. 1998). Furthermore, selective deletion in dopamine neurons of the Ret tyrosine kinase, which interacts with GFRα1 to initiate intracellular effects of GDNF, does not affect their adult number (Jain et al. 2006; Kramer et al. 2007), leaving unresolved the precise role of endogenous GDNF in dopamine neuron development.
Characterization of the role that GDNF plays is also made complex by the observation that GFRα1 is not only expressed in dopamine neurons, in conjunction with Ret (Widenfalk et al. 1997; Yu et al. 1998; Sarabi et al. 2001), but also in their target, the striatum (Cho et al. 2004). This post-synaptic expression raises the possibility that GFRα1 may serve GDNF signaling not only cell autonomous to dopamine neurons (‘in cis’), but also in a non-cell autonomous capacity (‘in trans’). The possibility that post-synaptic GFRα1 expression may function in trans was originally suggested by the observation that it is expressed in the target regions of some Ret-expressing systems where Ret is not intrinsically expressed (Yu et al. 1998) (see Saarma 2001 and Paratcha and Ledda 2008 for reviews). Clear effects of GFRα1 expression in trans on neurite outgrowth have been identified in vitro (Paratcha et al. 2001; Ledda et al. 2002). However, its functional role in vivo is dependent on context; while abrogation of expression of GFRα1 in trans does not affect development of enteric or motor neurons (Enomoto et al. 2004), it is essential for specific phenotypes of cortical GABAergic neuron (Canty et al. 2009).
Therefore, the present investigations sought to determine whether GFRα1 signaling in trans from post-synaptic expression in striatal neurons may regulate the development of mesencephalic dopamine neurons. In addition, while prior studies have focused exclusively on the role of post-synaptic expression in developmental contexts, we have herein examined its role in the response of adult dopaminergic neurons to injury. For these studies, we have created transgenic mice in which GFRα1 is expressed with regional and temporal selectivity in targets of the mesencephalic dopaminergic projection.
Materials and methods
Transgenic mice
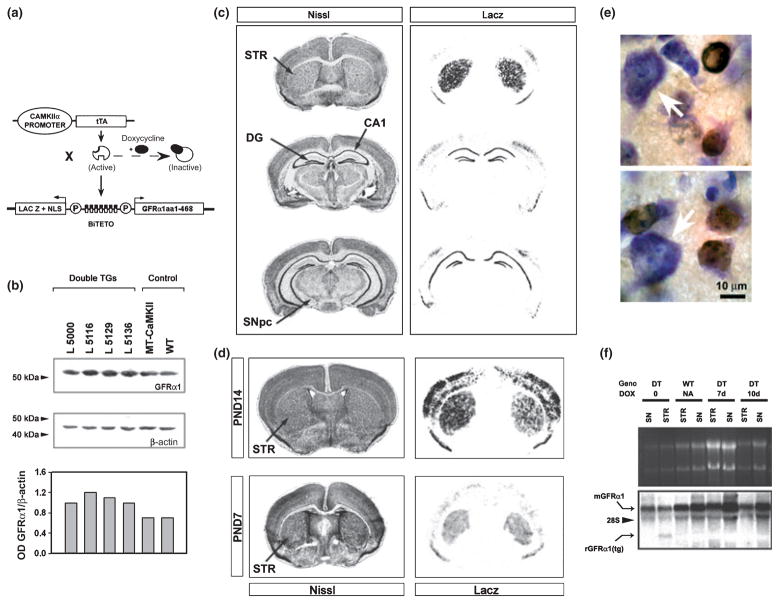
The coding sequence for rat GFRα1 was obtained by performing RT-PCR on RNA derived from postnatal rat brain, utilizing primers to encompass bps 129–1685 (Accession NM012959). This fragment was inserted into multiple cloning site 2 in the appropriate orientation downstream to minimal promoter Pmin-1 in the bidirectional plasmid pBI-3 (Baron et al. 1995) (Fig. 1a). An 8-kb fragment of this construct was microinjected by standard techniques into single cell CBA × C57BL/6 embryos. Four lines were obtained and expanded by mating with C57BL/6 inbred mice. In order to achieve regionally selective expression in projection targets of mesencephalic dopaminergic systems these transgenic mice were crossed with mice carrying a calcium/calmodulin-dependent protein kinase II-tetracycline-dependent transcription activator (CaMKII-tTA) transgene, as previously described (Mayford et al. 1996; Yamamoto et al. 2000) (Fig. 1a). These crosses revealed that all four BiTetO-LacZ-rGFRα lines, when crossed with the CaMKII-tTA line, produced double transgenic offspring with positive LacZ staining in brain. Doxycycline treatment of double transgenic mice was carried out as previously described (Yamamoto et al. 2000), and suppression of transgene expression was confirmed by Northern analysis, performed as described (Kholodilov et al. 1999).
Fig. 1.
Characterization of CaMKIIα-tTA × BiTetO-LacZ-rGFRα1 (CBL-GFRα1) mice. (a) Monotransgenic mice carrying the CaMKIIα-tTA transgene are crossed with mice carrying the BiTetO-LacZ-rGFRα1 transgene. The BiTetO promoter is bidirectional, and drives LacZ expression in one direction, and rat GFRα1 (aa1–468) in the other. (b) Four BiTetO-LacZ-rGFRα1 lines were obtained (Lines. 5000, 5116, 5129, 5136) and crossed with CaMKIIα-tTA mice. Double transgenic (TG) CBL-GFRα1 offspring were identified, and levels of striatal rGFRα1 protein expression, assessed by western analysis, were compared to levels in littermate monotransgenic (MT) CaMKIIα-tTA and non-transgenic (WT, wildtype) controls. A single representative analysis shows that all four lines expressed higher levels of GFRα1 protein than endogenous levels in controls. When the optical density of each band is normalized for the optical density of β-actin, Line 5116 demonstrated the highest density ratio, as shown in the bar graph. (c) In Line 5116, regional patterns of transgene expression are demonstrated by LacZ staining. Staining is observed in striatum (STR), cortex, and the dentate gyrus (DG) and CA1 region of the hippocampus. Notably, there is no staining in the SNpc. (d) LacZ expression is observed in the striatum (STR), cortex and hippocampus (not shown) at PND7 and 14. (e) Immunoperoxidase staining for β-galactosidase reveals expression within medium-size striatal neurons. Large striatal neurons (white arrows) were not stained. (f) Northern analysis of striatal (STR) and substantia nigra (SN) samples reveals mRNA for endogenous mouse GFRα1 (mGFRα1) in both double transgenic (DT) and non-transgenic, wildtype (WT) mice in both regions. A shorter mRNA species, of the expected size for the transgene encoding rat GFRα1 (aa1–468) [rGFRα1 (tg)], is observed exclusively in the striatum of the double transgenic mouse in the absence of doxycycline. In the presence of doxycycline treatment for 7 or 10 days, this mRNA derived from the transgene is no longer observed.
LacZ staining and β-galactosidase immunohistochemistry
For LacZ staining, brains were removed rapidly and quickly frozen in isopentane on dry ice. Sections 14 μm thick were cut in a cryostat, thaw-mounted on slides, and stored at −80°C. At the time of staining, the slides were warmed to 20–25°C, and the sections fixed in 4% paraformaldehyde/0.1 M phosphate buffer (PB). After a rinse in phosphate buffered saline (PBS), sections were incubated in X-gal staining solution as previously described (Kholodilov et al. 2004) at 37°C overnight. For demonstration of β-galactosidase expression at a cellular level, immunohistochemistry was performed. Animals were initially perfused with saline through a left intraventricular cannula by gravity, followed by 4% paraformaldehyde/0.1 M PB. Brains were then post-fixed in the same fixative for 24 h, and then cryoprotected in 20% sucrose for an additional 24 h at 4°C. The brains were then rapidly frozen in isopentane on dry ice and sections were cut in a cryostat at 20 μm. Sections were processed free-floating. Following a PBS wash, and treatment with PBS/0.5% bovine serum albumin/0.1% Triton, sections were incubated with rabbit anti-β-galactosidase (Cortex-Biochem, Madison, WI, USA) at 1: 500 for 48 h at 4°C. After a wash, sections were then incubated in biotinylated Protein A (prepared in this laboratory) at 1: 100 for 1 h between 20–25°C. Sections were then incubated with avidin-biotinylated-horseradish peroxidase complexes (ABC, Vector Laboratories, Burlingame, CA, USA) at 1: 600 for 1 h. After incubation with diaminobenzidine, sections were mounted onto subbed slides and counter-stained with thionin.
Western analysis of GFRα1
Individual striata were homogenized in lysis buffer, containing 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40 (Sigma-Aldrich, St Louis, MO, USA), 5 μg/mL aprotinin, 5 μg/mL leupeptin and 17 μg/mL phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 14 000 g at 4°C and the protein concentration of the supernatant was determined with a micro BCA kit (Pierce, Rockford, IL, USA). An aliquot containing 50–70 μg of protein per lane was diluted in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA), electrophoresed in a 15% polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The membrane was probed with anti-GFRα1 antibody (RDI, Concord, MA, USA) at 1: 500, treated with appropriate secondary antibodies, conjugated with horseradish peroxidase and detected with a chemiluminescent substrate (Pierce).
Immunohistochemistry for tyrosine hydroxylase in dopamine neurons of the substantia nigra
Animals were perfused with saline through a left intraventricular cannula by gravity, followed by 4% paraformaldehyde/0.1 M PB. The brains were removed and post-fixed in the same fixative for 1 week. Each brain was cryoprotected in 20% sucrose for 24–48 h, and rapidly frozen. A complete set of serial sections through the substantia nigra (SN) was cut at 30 μm. Sections were saved individually in serial order at 4°C and every fourth individual sections was selected for tyrosine hydroxylase (TH) immunostaining, in conformity with the fractionator method of sampling (Coggeshall and Lekan 1996) (see below). Sections were processed free-floating, as described above for β-galactosidase, with the exception that the primary antibody was a rabbit anti-TH (Calbiochem, San Diego, CA, USA) at 1: 1000. After treatment with biotinylated Protein A and ABC, sections were mounted on subbed slides in serial order, and thionin counter-stained.
Determination of SN and ventral tegmental area dopamine neuron numbers by stereologic analysis
A complete set of TH-immunostained serial sections, sampled as every fourth section through the SN, was analyzed by a stereologic method for each animal. Each analysis was performed under blinded conditions on coded slides. For each animal, the SN on one side of the brain was analyzed. For each section the entire SN was identified as the region of interest. Using StereoInvestigator software (Micro Bright Field, Inc., Williston, VT, USA) a fractionator probe was established for each section. The number of TH-positive neurons in each counting frame was determined by focusing down through the section, using 100× objective under oil, as required by the optical disector method (Coggeshall and Lekan 1996). Our criterion for counting an individual TH-positive neuron was the presence of its nucleus either within the counting frame, or touching the right or top frame lines (green), but not touching the left or bottom lines (red). The total number of TH-positive neurons for each SN on one side was determined by the StereoInvestigator program. The total volume of the SN was also determined by the StereoInvestigator program for each brain based on the sum of volumes derived from the area of each individual serial section and the tissue height represented by that section. In a separate analysis of the same sections used for the SN determinations, the number of neurons in the ventral tegmental area (VTA) was determined.
Striatal TH and dopamine transporter fiber immunostaining and analysis
For TH-positive fiber staining, each mouse was perfused by peristaltic pump at 10 mL/min via an intraventricular cannula with chilled 0.9% NaCl for 3 min followed by chilled 4% paraformaldehyde/0.1 M PB for 8 min. Each brain was post-fixed in the same fixative for 48 h at 4°C. Without cryoprotection, each brain was rapidly frozen in isopentane on dry ice. Coronal sections were cut through the striatum at 30 μm from planes 4.06–4.90 mm (interaural) (Paxinos and Franklin 2001) and processed free-floating. Following a wash in Tris-buffered saline (TBS), and treatment with TBS/5% goat serum, sections were incubated with rabbit anti-TH (Calbiochem) at 1: 500 for 48 h at 4°C. Sections were treated with biotinylated goat anti-rabbit (Vector Labs) at 1: 400 for 1 h between 20–25°C. They were then treated with ABC, and incubated with diaminobenzidine. The regional optical density of TH immunostaining over the striatum was determined as previously described (Burke et al. 1990). The optical density analysis was performed under blinded conditions on coded slides. For dopamine transporter immunostaining, each animal was perfused by pump at 20 mL/min intracardially with 0.1 M PBS (1 unit heparin/mL) at 37°C for 1.5 min, followed by chilled 4% paraformaldehyde/0.1 M PB by gravity for 5 min. The brain was removed, blocked, and post-fixed for 24 h. Sections were washed in TBS, treated with TBS/0.1% Triton, and blocked with 0.1 M TBS/3% normal rabbit serum. After another TBS wash, they were incubated with rat anti-dopamine transporter (Chemicon, Temecula, CA, USA) at 1: 1000 in TBS/3% normal rabbit serum for 48 h at 4°C. Sections were treated with biotinylated rabbit anti-rat (Vector) at 1: 300 for 1 h between 20–25°C, followed by treatment with ABC and then incubation with diaminobenzidine. The regional optical density of dopamine transporter immunostaining over the striatum was then determined.
Behavioral analysis
Spontaneous motor activity in adult mice was assessed by use of a Med Associates, Inc. Activity Monitor System (St. Albans, VT, USA), and expressed as distance traveled per 10-min epoch. Motor activity response to injection of amphetamine (2.0 mg/kg) was assessed in a separate group of animals by use of the following protocol: on Day 1, spontaneous motor activity over 2 h was assessed; on Days 2 and 3 motor activity was assessed over 2 h following administration of vehicle and amphetamine, respectively. Each animal was placed in the activity area immediately following injection at Time = 0. For spontaneous motor activity, two way ANOVA revealed no difference between males and females for either genotype, so data from males and females were combined.
Measurement of striatal dopamine and metabolites
For determination of striatal levels of dopamine and its metabolites, mice were decapitated, their brains rapidly removed, and placed into a chilled glass plate. The striatum on each side of the brain was punched out with a 2.0-mm punch, and immediately frozen on dry ice. For each pair of striata, dopamine, dihydroxyphenylacetic acid and homovanillic acid were determined by HPLC by Bioanalytical Systems Inc. (West Lafayette, IN, USA), and expressed as ng per sample.
Experimental 6-hydroxydopamine lesions
Adult (8–12 weeks) littermate mice were obtained from CaMKIIα-tTA × BiTetO-LacZ-rGFRα (CBL-GFRα1) crosses. For 6-hydroxydopamine (6-OHDA) experiments, non-transgenic (WT, wildtype), monotransgenic CaMKIIα-tTA and double transgenic mice were used. Mice received a unilateral intra-striatal injection of 6-OHDA as described (Silva et al. 2005). Briefly, mice were pre-treated with desipramine, anesthetized with ketamine/xylazine solution and placed in a stereotaxic frame. A solution of 6-OHDA (5.0 μg/μL in 0.9% NaCl/0.02% ascorbate) was injected by microliter syringe at a rate of 0.5 μL/min by pump for a total dose of 15.0 μg/3 μL. Injection was performed into the left striatum at coordinates AP: +0.09 cm; ML: +0.22 cm; DV: −0.25 cm relative to bregma. After a wait of 2 min, the needle was slowly withdrawn. This procedures was approved by the Columbia University Animal Care and Use Committee.
Preparation and administration of adeno-associated virus (AAV) Myr-Akt and AAV DN(PH)-Akt vectors
A plasmid encoding a 5′ src myristoylation signal in frame with mouse Akt1 was kindly provided by Dr. Thomas Franke (Franke et al. 1995; Ahmed et al. 1997). The myristoylated-Akt1 (Myr-Akt) sequence was modified to incorporate a FLAG-encoding sequence at the 3′ end and inserted into an AAV1 packaging construct as previously described (Olson et al. 2006; Ries et al. 2006). The genomic titer was 1.6 × 1012 viral genomes/mL. A plasmid encoding the pleckstrin homology (PH) domain of mouse Akt1 (dominant negative Akt) (DN-Akt) was also kindly provided by Dr. Thomas Franke (Songyang et al. 1997), and modified to incorporate a FLAG-encoding sequence at the 3′ end. The genomic titer was 1.8 × 1012 viral genomes/mL. Yellow fluorescent protein was subcloned into the same viral backbone and viral stocks were used at a titer of 2.1–9.1 × 1012. For AAV injection into the SN, mice were anesthetized with ketamine/xylazine solution and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) with a mouse adapter. The tip of 5.0 μL syringe (Agilent, Santa Clara, CA, USA) needle (26S) was inserted to stereotaxic coordinates AP: −0.35 cm; ML: +0.11 cm; DV: −0.37 cm, relative to bregma. These coordinates place the needle tip dorsal to the posterior SN. Viral vector suspension in a volume of 2.0 μL was injected at 0.1 μL/min over 20 min. After a wait of 5 min, the needle was slowly withdrawn. Successful transduction of dopamine neurons of the SN was confirmed histologically by double immunolabeling for FLAG and TH.
Results
Characterization of CBL-GFRα1 mice
In order to create mice in which GFRα1 is selectively expressed in the target regions of the mesencephalic dopaminergic projections, CaMKIIα-tTA mice (Mayford et al. 1996) were crossed with mice carrying the BiTetO-LacZ-rGFRα1 transgene (Fig. 1a). Four lines, when crossed with CaMKIIα-tTA monotransgenic mice, yielded double transgenic offspring that expressed higher levels of GFRα1 protein in striatum than endogenous levels observed in monotransgenic and non-transgenic littermate controls (Fig. 1b). Among these lines, the highest levels of GFRα1 protein expression in striatum were observed in Line 5116, so all further studies were performed in these mice. We confirmed regional selectivity of LacZ expression in Line 5116 (Fig. 1c). As anticipated, based on known regional patterns of CaMKIIα expression (Mayford et al. 1996; Yamamoto et al. 2000; Kholodilov et al. 2004), LacZ activity was observed in striatum, hippocampus and cortex (Fig. 1c). Importantly, no LacZ expression was observed in neurons of the SN pars compacta (SNpc) (Fig. 1c), which express high levels of endogenous GFRα1 and Ret tyrosine kinase mRNA (Treanor et al. 1996; Widenfalk et al. 1997; Glazner et al. 1998; Golden et al. 1998). It has previously been demonstrated that the bidirectional BiTetO promoter achieves a regionally correlated expression of the two coding sequences under its control (Krestel et al. 2001), which in this case would be LacZ and rGFRα1. We therefore conclude that in Line 5116 we have achieved increased expression of rGFRα1 in target structures of the mesencephalic dopaminergic projection. To ascertain whether trans-gene protein expression occurs during the critical postnatal period when developmental cell death occurs in dopamine neurons of the SNpc (Janec and Burke 1993; Oo and Burke 1997) we assessed LacZ expression and found that it occurs in striatum, cortex and hippocampus on postnatal days (PND) 7 and 14 (Fig. 1d). In the striatum β-galactosidase protein expression was demonstrated exclusively in medium-size neurons (Fig. 1e). Thus, transgenic GFRα1 protein expression occurs in the neurons that are also the site of production of endogenous GFRα1 protein in striatum (Cho et al. 2004). The CBL-GFRα1 system permits regulation of transgene expression by administration of doxycycline, as shown for Line 5116 mice (Fig. 1f).
We have been unable to submit BiTetO-LacZ-rGFRα Line 5116 to a central mouse repository for the reason that, as we maintained the line in the C57BL/6 background, the transgene underwent silencing because of methylation, demonstrated by methylation-dependent restriction analysis (Allen et al. 1990). This phenomenon, referred to as ‘repeat-induced transgene silencing’ (Garrick et al. 1998) has been previously described (Allen et al. 1990; Engler et al. 1991), and we have previously encountered the same problem in another BiTetO line, BiTetO-LacZ-rGDNF (Kholodilov et al. 2004; Shakya et al. 2005). We attempted to reverse the silencing by backcrosses into the inbred Swiss Webster strain (SJL, Charles River, Wilmington, MA, USA), and passage through both males and females, but without success. In addition to DNA methylation, other epigenetic processes have been proposed to mediate silencing of BiTetO transgenes (Zhu et al. 2007).
Post-synaptic expression of GFRα1 in the striatum regulates the development of SNpc dopamine neurons
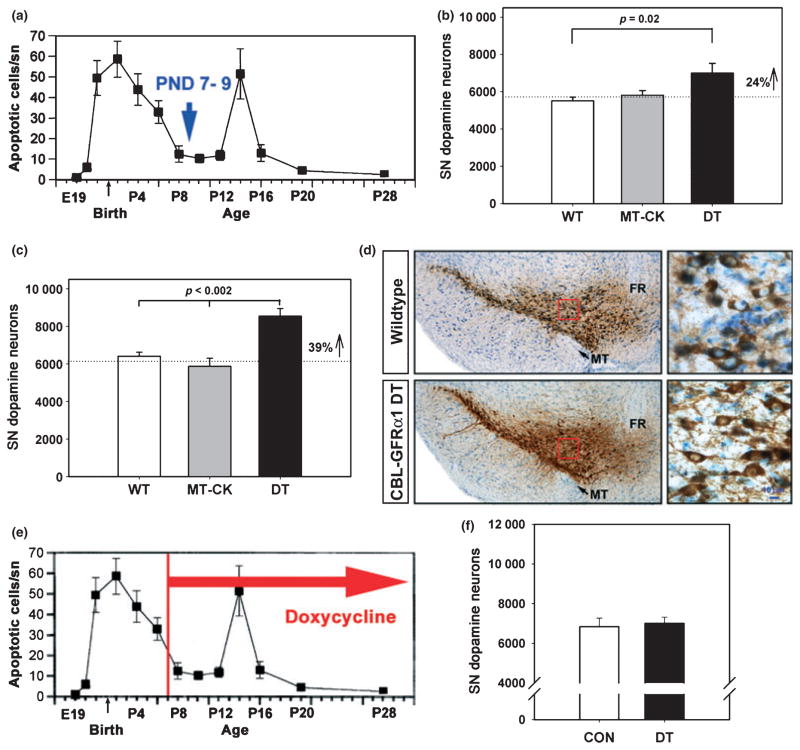
The postnatal developmental cell death event in SNpc dopamine neurons occurs in two phases, a first major phase peaking at PND2, and a second minor phase at PND14 (Fig. 2a). We therefore examined the effect of post-synaptic GFRα1 expression in striatum on the surviving number of SNpc dopamine neurons after the first phase, at PND7–9, and in adulthood, to determine effects on the final adult number. At PND7–9, post-synaptic striatal expression of GFRα1 resulted in a 24% increase in the number of SNpc dopamine neurons surviving the first phase of developmental cell death (Fig. 2b). In adulthood, this effect was more pronounced, observed as a 39% increase (Fig. 2c and d). Transgenic expression of GFRα1 in striatum had no effect on the number of dopamine neurons in the ventral tegmental area (A10) (Table 1), nor did it have an effect on SNpc dopamine neuron size. In spite of the increase in adult number of dopamine neurons, there was no effect on the morphology of striatal innervation, assessed as the density of both TH and dopamine transporter immunoperoxidase staining (Table 1).
Fig. 2.
Post-synaptic GFRα1 expression in striatum increases the number of SNpc dopamine neurons that survive developmental cell death. (a) Counts of the number of apoptotic profiles in the SNpc throughout the postnatal period reveal that there are two phases of developmental cell death. The first occurs just before and after birth; the second occurs at PND14. The data shown are derived from studies of rat (data from Oo and Burke 1997); a very similar time course has been demonstrated in mice (Jackson-Lewis et al. 2000). (b) After the first phase of developmental cell death (at PND7–9), striatal GFRα1 expression results in a 24% increase in the number of dopamine neurons in the SN (p = 0.02, ANOVA; n = 7, all groups) (WT, wildtype, non-transgenic littermate control; MT-CK, monotransgenic CaMK-tTA; DT, double transgenic CBL-GFRα1 mice). (c) The increased survival of SN dopamine neurons persists through the second phase of developmental cell death and into adulthood, when a 39% increase in their number is observed (p < 0.002, ANOVA, n = 6 for the WT and MT-CK groups; n = 8 for double transgenics). For this experiment, the ages of the mice ranged from 9 to 14 weeks. (d) Coronal sections of the SN have been immunoperoxidase stained for TH (brown) and Nissl counter-stained. Low power micrographs reveal an increased number of dopamine neurons in the CBL-GFRα1 DT mice (bottom left panel). Higher magnification of the regions outlined in red squares shows that there is no apparent difference in the size or morphology of the neurons (MT, medial terminal nucleus; FR, fasciculus retroflexus) (Bar = 10 μm). (e, f) Administration of doxycycline from PND7 into adulthood suppresses the effect of post-synaptic striatal GFRα1 on the surviving adult number of SN dopamine neurons.
Table 1.
Quantitative morphological analysis of mesencephalic dopaminergic systems in CBL-rGFRα1 mice
| Genotype | VTA
|
SN
|
Striatum
|
|||
|---|---|---|---|---|---|---|
| TH neuron number (n) | TH neuron size (μm2) (n) | Volume (μm3) | Size (mm2) | Optical density
|
||
| TH | DAT | |||||
| WT | 5529 ± 374 (6) | 172 ± 4.9 (120) | 844 ± 31 | 4.3 ± 0.2 | 0.10 ± 0.02 | 0.15 ± 0.02 |
| MT | 5312 ± 444 (6) | 164 ± 3.9 (120) | 793 ± 19 | – | – | – |
| DT | 5584 ± 408 (8) | 173 ± 3.3 (120) | 857 ± 37 | 4.0 ± 0.2 | 0.10 ± 0.01 | 0.12 ± 0.02 |
DT, double transgenic; MT, monotransgenic; SN, substantia nigra; VTA, ventral tegmental area, WT, wildtype.
Prior investigations of in trans effects of GFRα1 have noted a correlation with periods of maximal GFRα1 expression in tissue targets (Ledda et al. 2002). We have previously reported that the postnatal period of maximal expression of GFRα1 mRNA in striatal target is from PND10 to 14, corresponding to the second phase of developmental cell death (Cho et al. 2004). Given also that the effect of the transgene was augmented between PND7–9 and adulthood, we therefore sought to determine the role of post-synaptic GFRα1 expression during this period. In order to do this, we utilized the ability of doxycycline to suppress transgene expression (Fig. 1f). When doxycycline treatment is started on PND7 and continued until adulthood, an increase in the number of SNpc dopamine neurons is no longer observed (Fig. 2e and f). We therefore conclude that transgene expression during the second phase of cell death and into adulthood is necessary for the increase in dopamine neuron number.
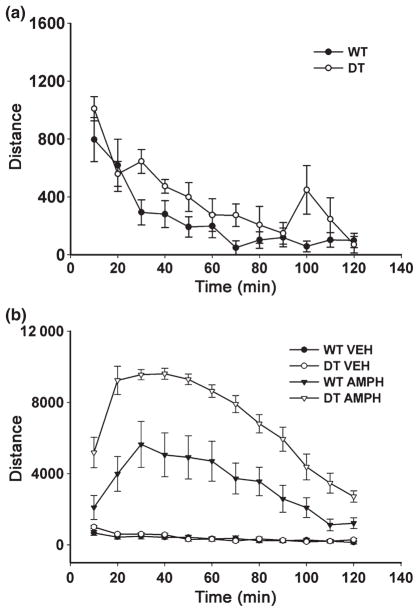
In spite of the increased number of dopamine neurons in the SN of adult double transgenic mice, there was no change in tissue levels of dopamine or its metabolites in the ventral mesencephalon (Table 2). In the striatum, in keeping with the absence of an effect on morphologic measures of dopaminergic innervation, there was no change in tissue levels of dopamine or its metabolites, or in the homovanillic acid/dopamine ratio, an index of dopamine turnover (Table 2). Despite the absence of effects on either morphologic or neurochemical indices of striatal dopaminergic innervation in the double transgenic mice, a motor behavior phenotype was observed. Double transgenic mice were not different from WT littermate control mice in their basal activity levels (Fig. 3a); however, following administration of amphetamine, activity levels were significantly greater in the double transgenic mice in comparison to the non-transgenic (WT) littermate controls (Fig. 3b). At 30–50 min after amphetamine injection, the time of peak effect for both genotypes, activity levels in the double transgenics were almost two-fold those observed in WT mice.
Table 2.
Analysis of dopamine and metabolites in mesencephalic dopaminergic systems of CBL-rGFRα1 mice
| Genotype | n | Ventral mesencephalon
|
Striatum
|
|||||
|---|---|---|---|---|---|---|---|---|
| DA | HVA | DOPAC | DA | HVA | DOPAC | HVA/DA | ||
| WT | 6 | 6.5 ± 0.3 | 3.9 ± 0.4 | 2.3 ± 0.1 | 105.8 ± 7.4 | 13.3 ± 1.2 | 7.6 ± 0.7 | 0.12 ± 0.01 |
| MT | 6 | 7.3 ± 1.5 | 3.9 ± 0.2 | 2.2 ± 0.2 | 96.2 ± 7.9 | 10.1 ± 1.3 | 7.3 ± 0.3 | 0.10 ± 0.01 |
| DT | 6 | 6.2 ± 0.6 | 3.6 ± 0.4 | 2.5 ± 0.2 | 93.3 ± 10.8 | 11.3 ± 1.4 | 7.4 ± 0.9 | 0.12 ± 0.01 |
DA, dopamine; DOPAC, dihydroxyphenylacetic acid; DT, double transgenic; HVA, homovanillic acid; MT, monotransgenic; WT, wildtype.
Fig. 3.
CBL-GFRα1 double transgenic mice have an increased behavioral response to amphetamine. (a) Both double transgenic (DT) and non-transgenic littermate (wildtype, WT) controls show a normal increased activity level when initially placed in the chamber (activity levels at Time = 10 and 20 min were significantly greater than levels at Time = 40 min and all subsequent times; p < 0.001 two-way repeated measures ANOVA; p < 0.05, all pairwise multiple comparison post hoc analysis; WT, n = 9; DT, n = 6). There was no genotype effect. (b) Following administration of amphetamine, there is a much greater degree of behavioral activation in the DT group (p = 0.001 for comparison of factor ‘genotype’, ANOVA, n = 8 both groups) (VEH, vehicle; AMPH, amphetamine).
Post-synaptic GFRα1 expression in striatum preserves striatal dopaminergic innervation in a model of neurotoxin-induced injury in adult mice
Having shown that transgenic GFRα1 expression in striatum modifies the development of dopamine neurons of the SN, we next sought to determine whether it also has effects on the neurobiology of these neurons in the adult context, particularly in response to injury. For these investigations we subjected these neurons to injury by intra-striatal injection of the neurotoxin 6-OHDA, a model that induces progressive neuron loss over several weeks (Sauer and Oertel 1994) because of apoptosis (Marti et al. 2002). Examination of the surviving nigral dopamine neurons at 4 weeks following 6-OHDA injection revealed no effect of GFRα1 expressed in the striatum (Fig. 4a). Surprisingly, in spite of the absence of preservation of neurons, there was a highly significant preservation of striatal dopaminergic innervation, measured as optical density of immunoperoxidase staining for TH on the lesioned side as a percent of the contralateral, non-lesioned side (Fig. 4b and c). By this measure double transgenic mice showed a 1.7-fold increase over values in monotransgenic control mice, mainly because of increase striatal dopaminergic innervation of the ventro-medial striatum (Fig. 4c).
Fig. 4.
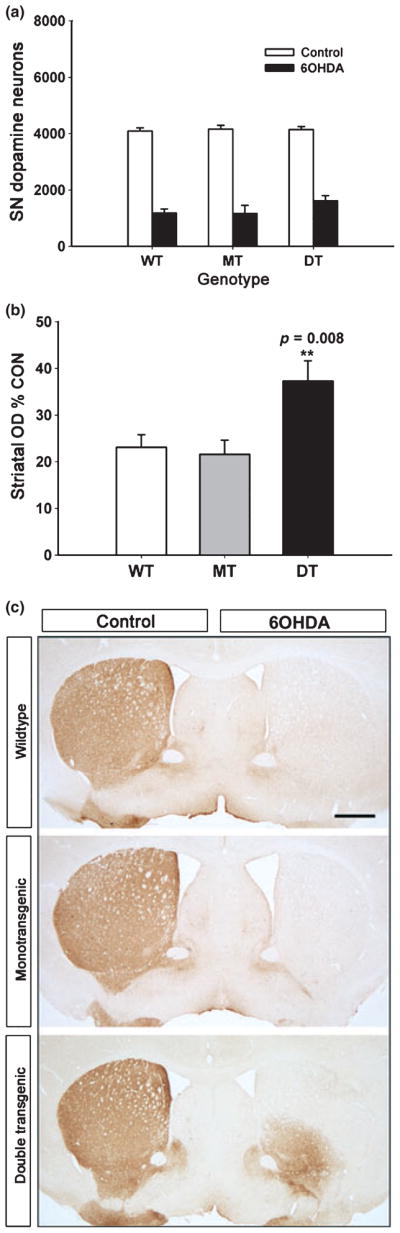
Preservation of striatal dopaminergic innervation following neurotoxin lesion by GFRα1 expression in trans. (a) Stereologic counts of dopamine neurons in the SN reveals no protective effect of striatal GFRα1 expression in the intra-striatal 6-OHDA lesion model (p > 0.05, all comparisons among 6-OHDA-treated conditions, ANOVA; n = 7, all groups). Adult (8–12 week) littermates of CaMKIIα-tTA × BiTetO-LacZ-rGFRα crosses were used, including non-transgenic (WT), monotransgenic (MT) (CaMKIIα-tTA) and double transgenic (DT) (CBL-GFRα1) genotypes. (b) In spite of the lack of protection of neurons, there is preservation of dopaminergic innervation, assessed as optical density of TH immunoreactivity. For this assessment, optical densities for the striatum on the lesioned side are normalized for the optical density of the contralateral, non-lesioned control side, and expressed as a percent of the control (p = 0.008, DT genotype vs. WT and MT, ANOVA; n = 7, all groups) (OD, optical density; CON, control). (c) Coronal sections of the striatum, immunoperoxidase stained for TH, show that the ability of GFRα1 to preserve innervation is primarily observed in the ventro-medial quadrant of the striatum. **p = 0.008.
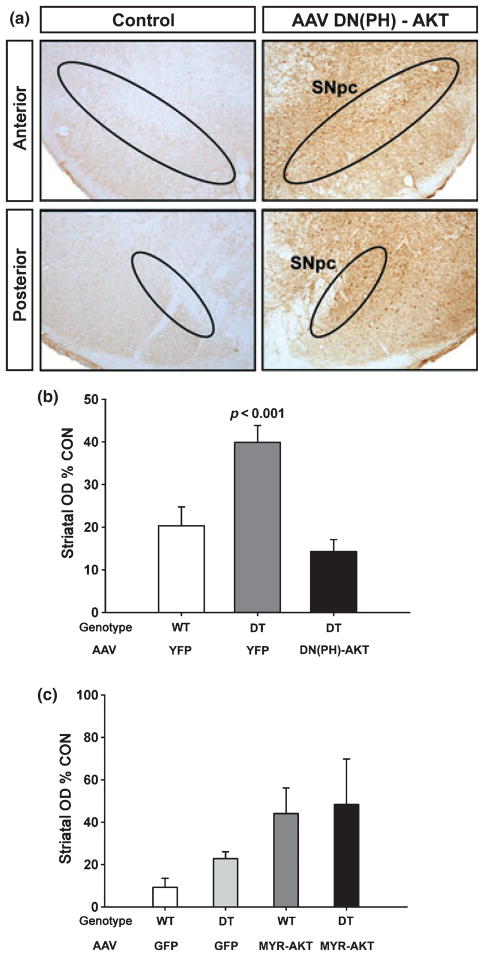
Previous experiments performed in tissue culture have suggested that an important cell signaling pathway in the mediation of GDNF effects through GFRα1 receptor binding is that of the phosphoinositide-3-kinase (PI3K)/AKT kinase cascade (Pong et al. 1998; Soler et al. 1999; Besset et al. 2000; De Vita et al. 2000; Sawada et al. 2000; Chen et al. 2001; Coulpier and Ibanez 2004), and we have recently shown that Akt signaling regulates dopamine neuron development in vivo (Ries et al. 2009). We therefore sought to determine whether this ability of post-synaptic expression of GFRα1 to preserve striatal innervation is mediated by PI3K/AKT signaling. We first examined the effect of a dominant negative form of Akt, consisting of its PH domain alone. The efficacy and specificity of this construct to inhibit Akt signaling has previously been demonstrated in vitro (Datta et al. 1995; Dudek et al. 1997; Songyang et al. 1997; Kim et al. 2002; Wang et al. 2003), and we have previously demonstrated its inhibitory effects on SN dopamine neuron development in vivo (Ries et al. 2009). Transduction of SN dopamine neurons with this dominant negative form of Akt abolished the ability of post-synaptic striatal GFRα1 to preserve striatal dopaminergic innervation following 6-OHDA lesion (Fig. 5b). We have previously shown that a constitutively active form of Akt, Myr-Akt preserves striatal dopaminergic innervation following 6-OHDA lesion (Ries et al. 2006). We therefore examined the effects of combining transduction of SN dopamine neurons with AAV Myr-Akt with transgenic striatal expression of GFRα1. As anticipated, either transduction with AAV Myr-Akt or transgenic striatal expression alone provided preservation of striatal innervation, but there was no additive effect when they were combined (Fig. 5c). Considering these results together with the ability of dominant negative Akt to block the effect of post-synaptic GFRα1, we conclude that its ability to preserve striatal innervation is likely to be mediated by Akt signaling.
Fig. 5.
Preservation of striatal dopaminergic innervation by GFRα1 expression in trans is regulated by Akt. (a) Successful transduction of SN neurons by AAV DN(PH)-Akt-FLAG is demonstrated by anti-FLAG immunoperoxidase staining. Left and right panels are taken from the same coronal section of the mesencephalon of an AAV-injected mouse. The side of AAV injection (right panels) shows abundant peroxidase labeling in the SN in both anterior (top panels) and posterior (bottom) planes. No FLAG staining is observed on the contra-lateral, non-injected side. (b) To assess the role of Akt signaling in the ability of GFRα1 to preserve striatal innervation either non-transgenic (WT) or double transgenic (DT) mice were first injected with a dominant negative, plekstrin homology domain alone form of Akt [DN(PH)-Akt], or yellow fluorescent protein (YFP) control. After 3 weeks, the mice were lesioned with 6-OHDA, and the extent of dopaminergic innervation of the striatum was assessed 4 weeks later. DN(PH)-Akt abrogated the ability of GFRα1 to preserve dopaminergic innervation [p > 0.05, DT/DN(PH)-AKT condition in comparison to WT/YFP, ANOVA; n = 6 for WT/YFP, n = 7 for DT/YFP, n = 8 for DT/DN(PH)-Akt]. Transduction of wildtype mice with DN(PH)-Akt had no effect on striatal dopaminergic innervation (data not shown) (OD, optical density; CON, control). (c) When double transgenic (DT) CBL-GFRα1 mice undergo transduction of their SN dopaminergic neurons with constitutively active Myr-Akt, there is no increase in the degree of striatal innervation provided by either transgene alone following 6-OHDA lesion (p > 0.05, WT/MYR-AKT in comparison to DT/MYR-AKT, ANOVA; n = 4 for WT/GFP, n = 5 for DT/GFP, n = 6 for WT/Myr-Akt, n = 5 for DT/Myr-Akt).
Discussion
We have previously shown that mesencephalic dopamine neurons depend on support from their target, the striatum, during postnatal development (Burke 2010), as envisioned by classic neurotrophic theory (Barde 1989). During the first two postnatal weeks, but not afterwards, axon-sparing ablation of the striatum results in an augmented developmental cell death event (Macaya et al. 1994; Kelly and Burke 1996) and fewer dopamine neurons surviving into adulthood (Burke et al. 1992). There is evidence that GDNF may serve as a striatal target-derived neurotrophic factor for these neurons during the first postnatal week. Intra-striatal injection of anti-GDNF neutralizing antibodies increases the level of apoptotic neuron death in SNpc during the first postnatal week, but not the second (Oo et al. 2003). Conversely, increased expression of GDNF selectively in the striatum in double transgenic mice results in an increased survival of SN dopamine neurons at PND7–9, after the first phase of developmental cell death (Kholodilov et al. 2004).
In spite of this evidence for regulation of the first phase of developmental cell death by striatal GDNF, increased expression of GDNF throughout the postnatal period is not sufficient to lead to an increase in the adult number of SN dopamine neurons (Kholodilov et al. 2004). We have therefore postulated that additional striatal-derived support of some kind is required during the second phase of developmental cell death, during the second postnatal week (Fig. 2a), in order to achieve lasting survival of an increased number of adult neurons.
We have observed that, like GDNF (Oo et al. 2005), GFRα1 mRNA is expressed in striatum during postnatal development, but with a different time course, being most abundantly expressed from PND10 to 14, during the second phase of developmental cell death (Cho et al. 2004). We noted with interest the results of Ledda et al., that developmental periods of maximal expression of GFRα1 mRNA in target correspond to periods of demonstrable in trans signaling effects (Ledda et al. 2002). We therefore considered the possibility that post-synaptic GFRα1 expression in the striatum may play a role in regulating postnatal developmental cell death in dopamine neurons, particularly the second phase at PND14. In support of this hypothesis, we herein find that increased expression of GFRα1 in the striatum results in an increased surviving number of these neurons at PND7–9, but most strikingly, a 39% increased in their final adult number. To determine whether post-synaptic striatal expression of GFRα1 after PND7 is necessary for the achievement of this increase in the adult number of dopamine neurons, we used doxycycline to suppress transgene expression after PND7. We find that suppression of the transgene abrogates the increase in the final adult number. We therefore conclude that increased GFRα1 expression in striatum throughout development is sufficient to increase the final number of mesencephalic dopamine neurons, and that expression after the first postnatal week is necessary for this effect.
Several possible mechanisms may account for the ability of transgenic striatal expression of GFRα1 to achieve a lasting increase in the adult number of SN dopamine neurons while a similar over-expression of GDNF in striatum does not (Kholodilov et al. 2004). As GFRα1 is relatively promiscuous among members of the GDNF receptor ligand family, it may interact with other members, such as neurturin (for review see Andressoo and Saarma 2008), and thereby achieve a more striking phenotype than that achieved by over-expression of GDNF alone. Alternatively, increased GFRα1 expression in the striatum may act in conjunction with GDNF or other family ligands to moderate apoptosis indirectly; for example, by regulating the number or strength of dopaminergic synapses (Ledda et al. 2002), and thereby augmenting alternate, non-GDNF related, striatum-derived neurotrophic support. One possible mechanism whereby GDNF may interact with GFRα1 to facilitate dopaminergic pre-synaptic development would be as a ligand-induced cell adhesion molecule, as recently described for hippocampal neurons (Ledda et al. 2007). Interestingly, in this regard, hippocampal GFRα1 mRNA is most highly expressed on PND10, as is striatal GFRα1 (Cho et al. 2004).
In future studies, it will be important to determine whether GFRα1 expressed post-synaptically in striatum is in a soluble or a membrane-bound state, because in trans effects have been demonstrated for both (Ledda et al. 2002). Such a difference in the mode of delivery would have important mechanistic implications, because a membrane-bound form would require direct post-synaptic target contact to mediate effects, whereas a soluble form would not.
To determine whether the lasting increase in the adult number of dopamine neurons induced by post-synaptic GFRα1 expression has functional correlates, we examined motor activity in double transgenic mice in comparison to WT littermate controls. While there was no significant effect of genotype on spontaneous motor activity, there was an approximately two-fold increase in the ability of amphetamine to increase motor activity. Our morphologic and biochemical studies of striatal dopaminergic innervation did not reveal changes that would account for this enhanced behavioral response. These studies, however, would not necessarily reveal important changes at the synaptic level or in the dynamics of dopamine release, so further analysis, including quantitative ultrastructural studies and investigations of the dynamics of dopamine release, will be required to define the neural basis of this behavioral phenotype in these mice.
While the precise role of GDNF in supporting SN dopamine neurons during development will require further study, there is no question that it is required for their survival in the adult, because genetic deletion results in gradual degeneration (Pascual et al. 2008). These observations raise questions about the source and nature of GDNF support of dopamine neurons not only in normal adult brain, but also in the context of injury and neurodegeneration. We therefore sought to determine in the CBL-GFRα1 mice whether striatal GFRα1 expression modifies the response of these neurons to injury. We observed that although striatal GFRα1 did not affect the number of surviving dopamine neurons, it provided a highly significant preservation of striatal dopaminergic innervation. Based on the well-known ability of GDNF to induce dopaminergic axon sprouting following injury (Hudson et al. 1995; Tomac et al. 1995; Rosenblad et al. 1998; Kirik et al. 2000; Kordower et al. 2000), we propose that this preservation may be because of an enhanced sprouting response. Delineation of the molecular basis of such an effect will require further investigation, although one possibility is that GFRα1 may act as a ligand-induced cell adhesion molecule in the adult brain to enhance dopaminergic terminal sprouting and differentiation (Ledda et al. 2007).
Surprisingly little is known about the intracellular signaling pathways utilized by the GDNF-GFRα1 interaction to mediate cellular effect on dopamine neurons in the in vivo context. The most abundant evidence derived from in vitro studies relates to activation of the Ret tyrosine kinase (reviewed in Airaksinen and Saarma 2002), followed by activation of a number of downstream mediators, including phospholipase Cc (Trupp et al. 1999), mitogen-activated protein kinase/extracellular signal-related kinase extracellular signal-related kinase 1/2 (Ugarte et al. 2003) and, most importantly, PI3K/AKT (Pong et al. 1998; Soler et al. 1999; Besset et al. 2000; De Vita et al. 2000; Sawada et al. 2000; Chen et al. 2001; Coulpier and Ibanez 2004). We have previously observed in both immature (Ries et al. 2009) and adult (Ries et al. 2006) rodents that transduction of SN dopamine neurons with a constitutively active form of Akt induces a robust sprouting response. In the developmental setting, a role for endogenous Akt in regulating neurite outgrowth is suggested by the ability of a dominant negative form of Akt to diminish innervation of the striatum (Ries et al. 2009). Based on these observations, we considered the possibility that Akt signaling may play a role in the ability of striatal GFRα1 expression to preserve striatal dopaminergic innervation following 6-OHDA lesion. In support of this possibility, inactivation of all three Akt isoforms by transduction of SN dopamine neurons with a dominant negative form [DN(PH)-AKT] completely abrogated the ability of GFRα1 to preserve innervation.
In conclusion, we have shown in CBL-GFRα1 double transgenic mice that GFRα1 post-synaptic expression in target striatum has a previously unrecognized ability to affect the neurobiology of SN dopamine neurons in vivo, both during development and in the setting of injury to the mature brain. These observations therefore provide a basis for the investigation of the role played by endogenous GFRα1 expression in striatum both on SN dopamine neuron development and their response to injury and disease.
Acknowledgments
This work was supported by NS26836, NS38370, and the Parkinson’s Disease Foundation. We gratefully acknowledge the assistance of Matt During and Chuansong Wang in the production of AAV vectors.
Abbreviations used
- 6-OHDA
6-hydroxydopamine
- AAV
adeno-associated virus
- CaMKII-tTA
calcium/calmodulin-dependent protein kinase II-tetracycline-dependent transcription activator
- CBL-GFRα1
CaMKIIα-tTA-BiTetO-LacZ-rGFRα
- GDNF
glial cell line-derived neurotrophic factor
- GFRα1
GNDF family receptor alpha-1
- Myr-Akt
myristoylated-Akt1
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PI3K
phosphoinositide-3-kinase
- PH
pleckstrin homology
- PND
postnatal day
- SN
substantia nigra
- SNpc
SN pars compacta
- TBS
Tris-buffered saline
- TH
tyrosine hydroxylase
- WT
wildtype
References
- Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Allen ND, Norris ML, Surani MA. Epigenetic control of transgene expression and imprinting by genotype-specific modifiers. Cell. 1990;61:853–861. doi: 10.1016/0092-8674(90)90195-k. [DOI] [PubMed] [Google Scholar]
- Alonso-Vanegas MA, Fawcett JP, Causing CG, Miller FD, Sadikot AF. Characterization of dopaminergic midbrain neurons in a DBH:BDNF transgenic mouse. J Comp Neurol. 1999;413:449–462. doi: 10.1002/(sici)1096-9861(19991025)413:3<449::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Andressoo JO, Saarma M. Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr Opin Neurobiol. 2008;18:297–306. doi: 10.1016/j.conb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Baron U, Freundlieb S, Gossen M, Bujard H. Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res. 1995;23:3605–3606. doi: 10.1093/nar/23.17.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besset V, Scott RP, Ibanez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Burke RE. Faactors shaping later stages of dopamine neuron development. In: Iversen LL, Iversen SD, Dunnett SB, Bjorklund A, editors. Dopamine Handbook. Oxford University Press; New York, NY: 2010. pp. 160–176. [Google Scholar]
- Burke RE, Cadet JL, Kent JD, Karanas AL, Jackson Lewis V. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: relationship to apo-morphine-induced rotations in 6-hydroxydopamine lesioned rats. J Neurosci Methods. 1990;35:63–73. doi: 10.1016/0165-0270(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Burke RE, Macaya A, DeVivo D, Kenyon N, Janec EM. Neonatal hypoxic-ischemic or excitotoxic striatal injury results in a decreased adult number of substantia nigra neurons. Neuroscience. 1992;50:559–569. doi: 10.1016/0306-4522(92)90447-a. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty AJ, Dietze J, Harvey M, Enomoto H, Milbrandt J, Ibanez CF. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J Neurosci. 2009;29:10695–10705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Chai Y, Cao L, Huang A, Cui R, Lu C, He C. Glial cell line-derived neurotrophic factor promotes survival and induces differentiation through the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathway respectively in PC12 cells. Neuroscience. 2001;104:593–598. doi: 10.1016/s0306-4522(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Cho JW, Yarygina O, Kholodilov N, Burke RE. Glial cell line-derived neurotrophic factor receptor GFRá-1 is expressed in the rat striatum during postnatal development. Mol Br Res. 2004;127:96–104. doi: 10.1016/j.molbrainres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Coulpier M, Ibanez CF. Retrograde propagation of GDNF-mediated signals in sympathetic neurons. Mol Cell Neurosci. 2004;27:132–139. doi: 10.1016/j.mcn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Datta K, Franke TF, Chan TO, Makris A, Yang SI, Kaplan DR, Morrison DK, Golemis EA, Tsichlis PN. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 2000;60:3727–3731. [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Engler P, Haasch D, Pinkert CA, Doglio L, Glymour M, Brinster R, Storb U. A strain-specific modifier on mouse chromosome 4 controls the methylation of independent transgene loci. Cell. 1991;65:939–947. doi: 10.1016/0092-8674(91)90546-b. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EMJ, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Hughes I, Golden J, Baloh RH, Yonemura S, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. GFRalpha1 expression in cells lacking RET is dispensable for organogenesis and nerve regeneration. Neuron. 2004;44:623–636. doi: 10.1016/j.neuron.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Garrick D, Fiering S, Martin DI, Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18:56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EMJ. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L, Lile JD, Collins F. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Iversen SD, Dunnett SB, Bjorklund A. Dopamine Handbook. Oxford University Press; New York, NY: 2010. [Google Scholar]
- Jackson-Lewis V, Vila M, Djaldetti R, Guegan C, Liberatore G, Liu J, O’Malley KL, Burke RE, Przedborski S. Developmental cell death in dopaminergic neurons of the sub-stantia nigra of mice. J Comp Neurol. 2000;424:476–488. doi: 10.1002/1096-9861(20000828)424:3<476::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM, Jr, Milbrandt J. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci. 2006;26:11230–11238. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janec E, Burke RE. Naturally occurring cell death during postnatal development of the substantia nigra of the rat. Mol Cell Neurosci. 1993;4:30–35. doi: 10.1006/mcne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Kelly WJ, Burke RE. Apoptotic neuron death in rat sub-stantia nigra induced by striatal excitotoxic injury is developmentally dependent. Neurosci Lett. 1996;220:85–88. doi: 10.1016/s0304-3940(96)13216-x. [DOI] [PubMed] [Google Scholar]
- Kholodilov NG, Neystat M, Oo TF, Lo SE, Larsen KE, Sulzer D, Burke RE. Increased expression of rat synuclein1 in the substantia nigra pars compacta identified by differential display in a model of developmental target injury. J Neurochem. 1999;73:2586–2599. doi: 10.1046/j.1471-4159.1999.0732586.x. [DOI] [PubMed] [Google Scholar]
- Kholodilov N, Yarygina O, Oo TF, Zhang H, Sulzer D, Dauer WT, Burke RE. Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J Neurosci. 2004;24:3136–3146. doi: 10.1523/JNEUROSCI.4506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestel HE, Mayford M, Seeburg PH, Sprengel R. A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res. 2001;29:E39. doi: 10.1093/nar/29.7.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Ibanez CF. Target-derived GFRalpha1 as an attractive guidance signal for developing sensory and sympathetic axons via activation of Cdk5. Neuron. 2002;36:387–401. doi: 10.1016/s0896-6273(02)01002-4. [DOI] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- Macaya A, Munell F, Gubits RM, Burke RE. Apoptosis in substantia nigra following developmental striatal excitotoxic injury. Proc Natl Acad Sci USA. 1994;91:8117–8121. doi: 10.1073/pnas.91.17.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti MJ, Saura J, Burke RE, Jackson-Lewis V, Jimenez A, Bonastre M, Tolosa E. Striatal 6-hydroxydopamine induces apoptosis of nigral neurons in the adult rat. Brain Res. 2002;958:185–191. doi: 10.1016/s0006-8993(02)03694-6. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Oo TF, Burke RE. The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Dev Brain Res. 1997;98:191–196. doi: 10.1016/s0165-3806(96)00173-3. [DOI] [PubMed] [Google Scholar]
- Oo TF, Kholodilov N, Burke RE. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal GDNF in vivo. J Neurosci. 2003;23:5141–5148. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo TF, Ries V, Cho J, Kholodilov N, Burke RE. Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat. J Comp Neurol. 2005;484:57–67. doi: 10.1002/cne.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo TF, Marchionini DM, Yarygina O, O’Leary PD, Hughes RA, Kholodilov N, Burke RE. Brain-derived neurotrophic factor regulates early postnatal developmental cell death of dopamine neurons of the substantia nigra in vivo. Mol Cell Neurosci. 2009;41:440–447. doi: 10.1016/j.mcn.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2001. [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pong K, Xu RY, Baron WF, Louis JC, Beck KD. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. J Neurochem. 1998;71:1912–1919. doi: 10.1046/j.1471-4159.1998.71051912.x. [DOI] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland RJ, During MJ, Kholodilov N, Burke RE. Oncoprotein Akt/PKB: trophic effects in murine models of Parkinson’s Disease. Proc Natl Acad Sci USA. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Cheng HC, Baohan A, Kareva T, Oo TF, Rzhetskaya M, Bland RJ, During MJ, Kholodilov N, Burke RE. Regulation of the postnatal development of dopamine neurons of the substantia nigra in vivo by Akt/protein kinase B. J Neurochem. 2009;110:23–33. doi: 10.1111/j.1471-4159.2009.06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Bjorklund A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson’s disease. Neuroscience. 1998;82:129–137. doi: 10.1016/s0306-4522(97)00269-8. [DOI] [PubMed] [Google Scholar]
- Saarma M. GDNF recruits the signaling crew into lipid rafts. Trends Neurosci. 2001;24:427–429. doi: 10.1016/s0166-2236(00)01864-6. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Hoffer BJ, Olson L, Morales M. GFRalpha-1 mRNA in dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area. J Comp Neurol. 2001;441:106–117. doi: 10.1002/cne.1400. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigro-striatal dopamine neurons following intrastriatal terminal lesions with 6 hydroxydopamine a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Nakanishi M, Akaike A, Shimohama S. Neuroprotective mechanism of glial cell line-derived neurotrophic factor in mesencephalic neurons. J Neurochem. 2000;74:1175–1184. doi: 10.1046/j.1471-4159.2000.741175.x. [DOI] [PubMed] [Google Scholar]
- Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, Burke R, D’Agati V, Costantini F. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. doi: 10.1016/j.ydbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord moto-neurons. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LFH, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Treanor JJS, Goodman L, deSauvage F, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Scott R, Whittemore SR, Ibanez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. J Neurosci Res. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor-beta (GDNFR-beta), novel proteins related to GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J Neurosci. 1997;17:8506–8519. doi: 10.1523/JNEUROSCI.17-21-08506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R. Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci. 1998;18:4684–4696. doi: 10.1523/JNEUROSCI.18-12-04684.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Aller MI, Baron U, et al. Silencing and un-silencing of tetracycline-controlled genes in neurons. PLoS ONE. 2007;2:e533. doi: 10.1371/journal.pone.0000533. [DOI] [PMC free article] [PubMed] [Google Scholar]