Abstract
Vocalizations emitted within a social context can trigger call-specific changes in the emotional and physiological/autonomic state of the receiver. The amygdala is implicated in mediating these changes, but its role in call perception remains relatively unexplored. We examined call and pitch selectivity of single neurons within the basolateral amygdala (BLA) by recording spiking activity in response to 5 pitch variants of each of 14 species-specific calls presented to awake, head-restrained mustached bats, Pteronotus parnellii. A response-wise analysis across neurons revealed seven types of temporal response patterns based on the timing and duration of spiking. Roughly half of the responses to different call types were significantly affected by changes in call pitch. A neuron-wise analysis revealed that ∼12% (8/69) of the neurons preferred the same pitch across all call types. Ninety-three percent (93/100) of neurons were excited by at least one call type and 76% exhibited either complete or transient suppression to one or more call types. The majority of neurons preferred fewer than half of the 14 different simple-syllabic calls. A call-wise analysis of spiking activity revealed that call types signaling either threat or fear most consistently evoked increases in the spike rate. In contrast, calls emitted during appeasement tended to evoke spike suppression. Our data suggest that BLA neurons participate in the processing of multiple call types and exhibit a rich variety of temporal response patterns that are neither neuron nor call specific.
Keywords: auditory system, affect, communication, pitch perception, valence, neural coding
audiovocal communication is among the most important means of transmitting information between conspecifics in species ranging from insects to birds and mammals (Ehret and Bernecker 1986; Ghazanfar and Hauser 2001; Hoy 1978; Janik 2000; Konishi 1994; Machens et al. 2001; Nelson and Marler 1989; Wilson and Hare 2004). Conspecific communication sounds or “calls” represent a special class of motivationally salient stimuli that are rich in acoustic structure (Kanwal et al. 1994; Ma et al. 2006; Morton 1977) are readily discriminated (Bastian and Schmidt 2008; Blumstein and Daniel 2004; Esser and Lud 1997; Hauser 1998) and convey information about the intentional state of the emitter (Ghazanfar and Hauser 1999; Litvin et al. 2007; Morton 1977; Seyfarth and Cheney 2003). Calls often modify the autonomic state and overt behavior of the receiver (Berntson and Boysen 1989; Hammerschmidt et al. 2009; Wilson and Hare 2004; Wohr and Schwarting 2007). Auditory processing for audiovocal communication has been largely studied within the lemniscal (ascending auditory) pathway for an understanding of the neural representation of individual acoustic features. This includes special mechanisms for an analysis of neuroethologically relevant natural sounds (Fitzpatrick et al. 1993; Kanwal et al. 1999; Manabe et al. 1978; Suga 1994; Suga et al. 1987, 1979), such as echolocation in bats, and general mechanisms for the processing and perception of the acoustic scene and its statistical structure (Fishman et al. 2001; Steinschneider et al. 2005). Here we use species-specific social calls to establish the amygdala as a call processing center in the mammalian brain.
The basolateral amygdala (BLA) is a major part of a proposed “fronto-temporal” system (Swanson and Petrovich 1998). The BLA consists of a cytoarchitecturally, functionally, and hodologically similar set of nuclei that receive inputs from the thalamus, hippocampus, and cortex (Davis and Whalen 2001; Pitkanen 2000). In the terminology adopted here, the BLA includes the lateral nucleus (LN) and basal nuclei (BN). Neuroanatomical and behavioral studies in rats and imaging studies in humans show that the amygdala is activated by auditory inputs (Muramoto et al. 1993; Nader et al. 2001; Romanski et al. 1993; Sander et al. 2003; Sander and Scheich 2001, 2005). In rats, neurons of the medial and dorsal divisions of the medial geniculate body, which receive nonlemniscal inputs, also project to the amygdala (Doron and Ledoux 2000; Edeline and Weinberger 1992; LeDoux et al. 1985). The BLA receives inputs from the frontal cortex and nonprimary auditory cortices (including “association” areas; see Fig. 1A) (McDonald 1998; Pitkanen et al. 2000; Romanski and LeDoux 1993; Stefanacci and Amaral 2002). Some studies suggest that the LN also receives weak to moderate direct projections from the primary auditory cortex (AC) (Budinger et al. 2008; Fitzpatrick et al. 1998). Furthermore, BLA receives afferents carrying information about intrinsically aversive and rewarding stimuli (Lanuza et al. 2004; Loughlin and Fallon 1983; McDonald 1998), which can be associated with environmental sounds as well as acoustic features within calls through experimental conditioning (Ma et al. 2010) or natural experience. The amygdala's role in aversive and appetitive conditioning is well established (Everitt et al. 2000; Maren and Quirk 2004; Salzman et al. 2007).
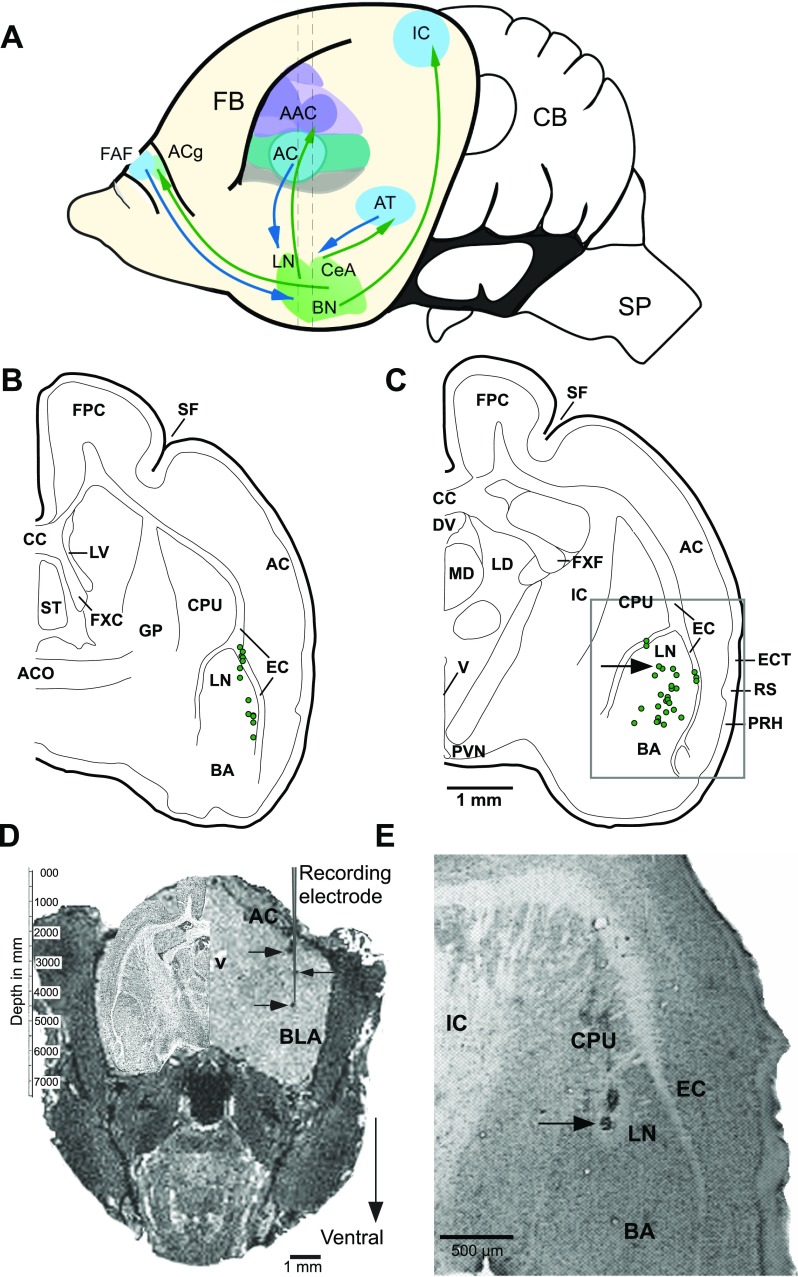
Fig. 1.
A. An outline of the mustached bat brain illustrating reciprocal connections between the amygdala and structures in the auditory forebrain of mammals (Armony et al. 1997; LeDoux et al. 1990a,b). Basolateral amygdala (BLA) also receives projections from the dorsomedial prefrontal cortex (McDonald et al. 1996) where a frontal auditory field (FAF) is present in this species. Central nucleus of the amygdala (CeA) projects back to the anterior cingulate (ACg) (Ghashghaei and Barbas 2002), and basal nuclei (BN) projects to the inferior colliculus (IC) within the brainstem (Marsh et al. 2002). Vertical dashed lines indicate the antero-posterior levels of the sections charted in B and C, respectively. B and C: reconstruction of locations of 55 recorded units from 48 different sites in the basolateral complex of the amygdala. Units recorded from the left (12) and right (43) hemispheres at each rostrocaudal level are plotted on the same hemisection. Two sections are separated by ∼600 μm along the rostrocaudal axis. Recording sites were located within 300 μm of the section plane. Grey rectangular outline in C shows location of the image in E. D: digitally sectioned coronal image of a bat brain with dark spots (on right side) indicating iron deposited in an electrode track and visualized using MRI procedure (grey vertical line representing a recording electrode is superimposed to emphasize collinearity of deposits). Iron deposits were made at depths of 1.5, 3.0, and 3.75 mm from the skull surface with anodal current levels of 2, 2, and 3 μA, respectively. Current duration ranged from 15 to 20 s. A high contrast Nissl-stained section from equivalent anteroposterior level of the brain is superimposed in the cranial space on the left. See text for imaging parameters. Recordings were subsequently performed in this animal. E. photomicrograph of a Nissl-stained coronal section showing a lesion mark at one of the recording sites. Arrow in D indicates the position of the recovered lesion indicated in C, projected caudally onto the schematic. AC, anterior cortex; AAC, auditory association cortex; ACO, anterior commissure; AT, auditory thalamus; BA, basal nucleus of the amygdala; CB, cerebellum; CPU, caudate putamen; CC, corpus callosum; DV, dorsal third ventricle; EC, external capsule; ECT, ectorhinal cortex; FB, forebrain; FPC, frontoparietal cortex; FXC, column of the fornix; FXF, fimbria of the fornix; GP, globus pallidus; LD, lateral dorsal nucleus of the thalamus; LN, lateral nucleus; LV, lateral ventricle; MD, mediodorsal nucleus of the thalamus; PRH, perirhinal cortex; PVN, paraventricular nucleus; RS, rhinal sulcus; SF, sylvian fossa; SP, spinal cord; ST, septal triangular nucleus; V, ventricle.
It is well known that the amygdala participates in creating associations between environmental stimuli and danger in the immediate environment (LeDoux 2003). We propose that a basic function of the amygdala in all vocal species is also to motivate the receiver to respond appropriately to social vocalizations. For this to occur, BLA neurons are expected to respond selectively to species-specific calls. Extensive processing of social calls in the amygdala may even shape call selectivity, receptive field plasticity, and temporal firing patterns of neurons in the AC and thalamus. Similarly, projections from the magnocellular BN of the amygdala to the inferior colliculus may gate the transmission of ascending acoustic information (Marsh et al. 2002).
Mustached bats are highly social and vocal and live in colonies of several thousand individuals. The social calls emitted by this species have been studied extensively (Kanwal et al. 1994), and the responses of neurons in the auditory and frontal cortices have been well documented (Kanwal et al. 2000, 1994). A digitized library of thousands of mustached bat calls is available, and call variants have been synthesized that capture variations in pitch that match the natural variation in each call type (Supplemental Fig. S1A; Supplemental Material for this article is available online at the J Neurophysiol website) (Ohlemiller et al. 1994). Recent behavioral studies have also established the association of simple syllabic calls to aggressive, fearful, and affiliative social behaviors (Clement et al. 2006). Here we tested the BLA's role in processing 14 different call types and their pitch-shifted variants presented at different amplitudes. We recorded well-isolated single unit activity in the BLA in response to these socially relevant natural sounds. These data provided clues to the extent and nature of auditory representation present within the amygdala. We examine call-evoked temporal response patterns and how they related to each other independent of neuron and call identity, the representation of call types within the BLA in terms of both response enhancement and suppression of spontaneous activity, and the selectivity of neurons for pitch and for call type. Finally, we examine if some response features show a spatial organization within the BLA. Our analyses of single neuron call responses provide a deeper understanding of how sounds are represented and processed within the amygdala.
MATERIALS AND METHODS
Experimental animals.
Wild mustached bats were caught in Trinidad. The mean body weight of the animals was ∼20 g. Bats were housed under diurnal lighting conditions (dimmed lights from 0900 to 1400), and deionized water and mealworms were provided ad libitum. Environmental temperature was maintained at ∼27°C and relative humidity at near 60%. Biosafety level II procedures were followed for all animal handling and experimental protocols per guidelines established by the Centers for Disease Control.
Surgery.
Bats were administered medetomidine (0.3 mg/kg) as a preanesthetic. For anesthesia, an initial dose of 0.5 to 1% isoflurane was supplied for 1 to 2 min, or until the animal ceased to respond to sound or pinching of the foot, and was followed by a continuous stream of ∼0.25% isoflurane/oxygen mixture. The animal's respiration was maintained at an average rate of ∼70 breaths per minute and temperature at ∼34° C throughout the surgery. A skin incision was made at the mid-line on the head and a metal post (15-mm long and 2-mm diameter) was affixed to the skull with cyanoacrylate (Loctite 411, Loctite) or methacrylate (OptiBond self-etching dental adhesive, cured with the Demetron A2 light-curing unit, both from Kerr). The bat was allowed to recover for a minimum of 3 days before the first recording session. Recording sessions lasted from 5 to 8 h a day and were performed two or three times a week for a period of up to 12 wk. Surgical and experimental procedures were approved by the Georgetown University Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3282-01).
Acoustic stimulation.
Experiments were conducted inside a heated (31°C) soundproofed chamber (IAC 400A) lined with echo-attenuating foam (Sonex, Acoustical Solutions). Tones were presented from two custom-made condenser loudspeakers, and call stimuli were delivered via a leaf-tweeter (Panasonic model #841). The three speakers were positioned at the same azimuth, 95 cm directly in front of the bat, and were calibrated with a microphone (ACO Pacific) placed at the position of the ear. The output of each speaker was reasonably flat (±6 dB) between 5 and 60 kHz for the leaf tweeter and 10 to 100 kHz for the condenser speakers.
An analog waveform generator (Wavetek 144) and custom hardware produced constant frequency (CF or “pure tone”) bursts of 30-ms duration with a 0.5-ms onset and offset taper. A series of tones stepped up and down in frequency and attenuated by 10 dB after 20 repetitions at each amplitude level were used to identify the best amplitude at a neuron's best frequency. Tone amplitudes ranged between 55 and 75 dB SPL.
Species-specific call stimuli included 14 simple-syllabic call types ranging in duration from 4 to 167 ms. Variants of each call type were generated by shifting the “pitch,” or fundamental frequency, by either ±1 or ±2 SD of the mean across all exemplars of a given call type. This procedure was used to produce multiple sets of each of the 14 calls, collectively representing the natural variation in pitch among recorded examples of each type. Details of stimulus conditioning for presentation are explained elsewhere (Kanwal et al. 1994; Ohlemiller et al. 1994). Briefly, simple syllabic calls were digitized and presented at a 250-kHz sampling rate using SIGNAL software (Engineering Design) and an A/D-D/A board (DT 2821G). Call presentation was sequenced according to an acoustic classification of call types via multidimensional scaling to a single dimension.
During single-unit recordings, calls were presented at a rate of either one or two per second to minimize adaptation effects. A no-stimulus control (silence) preceded presentation of an array of different call types. Call stimuli were attenuated by either 5 or 10 dB after 20 repetitions (trials) of the entire array. This procedure was repeated for each set of pitch-shifted variants of the fundamental frequency of each call type. This yielded responses to 14 call types at 3 to 5 intensity levels for 3 to 5 fundamental frequency or pitch-shifted variants (i.e., typically between 42 and 70 different call stimuli). In a few cases, call types were presented in random order and with randomized interstimulus intervals to rule out any effects of presentation order or short-term response history of a neuron.
Recording of neural activity.
The activity of single neurons was recorded with sharpened, vinyl-coated tungsten-wire electrodes with tip diameters of ∼10 μm and impedance of 1.5 to 3 MΩ. Stainless steel electrodes (FHC, Bowdoin, ME) with impedances of 3–4 MΩ were also used for recording and to deposit iron at recording locations at the end of several recording sessions in each of three animals. A reference electrode was placed in the nonauditory frontal cortex. In addition, an electrode bundle of 5–10 wires of either tungsten or nickel-chromium (with 10- and 15-μm wire diameters, respectively, and with one wire serving as a reference input) were also used to record spiking activity.
Neural activity from single electrodes was amplified with a Grass P55 preamplifier, and signals from wire bundles were amplified using a 16-channel high impedance headstage and a preamplifier (Tucker Davis Technologies RA16AC and RA16PA). Single unit acitivity was bandpass filtered between 500 and 3,000 Hz. Local field potentials were digitized at 1–5 kHz. Multi/single unit (MU/SU) activity was digitized at >20 kHz, and threshold crossing and template matching were used for online spike sorting using a Power 1401 and Spike2 software (Cambridge Electronic Design). Raster plots, peristimulus histograms, and average evoked local field potentials were generated online for a visual representation of responses. An audio-monitor and oscilloscope also aided in the audiovisual identification of stimulus-evoked responses along the trajectory of the electrode.
Calls were presented as search stimuli as the electrode was advanced remotely and neural activity was continuously monitored. Locations of rapidly adapting neurons were noted, but no further attempt was made to study them. If any isolated unit responded consistently to call stimuli, preference for different call types, including one or more pitch-shifted variants, was tested.
Stereotaxic method and recording site reconstruction.
The LN and BN of the amygdala were targeted using an unpublished atlas of the mustached bat brain (provided by William O'Neill) and measurements from Nissl-stained coronal sections (Prasada Rao and Kanwal 2004). Our stereotaxic method and apparatus are similar to those outlined by Schuller et al. (1986). During neural recordings, unanaesthetized bats were placed in a Styrofoam body mold to prevent excessive bodily movement. The head-post was fixed in the same position and orientation across recording sessions relative to the axes of three perpendicular micromanipulators (Mitutoyo Digimatic 164 series) and the leaf-tweeter speaker. Recording site coordinates were noted relative to a common zero point on the stereotaxic apparatus across recording sessions.
MRI and histology.
MRI was used to visualize iron deposits made with a stainless-steel electrode in one animal. After recording neural activity, anodal current (3 μA for 15 s) passed through the exposed tip of the recording electrode deposited a minute amount of iron at the recording location (Fung et al. 1998). For imaging, the animal's head was restrained using a custom-built bite-bar type holder, which was mounted to a sliding platform and positioned inside of a 30-mm inner diameter radio frequency volume coil (transmit and receive antenna) for noninvasive imaging of the bat brain (Fricke et al. 2004; Kamada et al. 1999). The animal was anesthetized with isoflurane in an oxygen/nitrous mixture. MRI was performed using a 7-Tesla small-animal magnet (Bruker-Biospin, Billerica, MA). The MRI pulse protocol was a rapid acquisition with relaxation enhancement (RARE) imaging sequence, TE 5.9, TR 200, Rare Factor 8, Matrix 256 × 256 × 256-points, FOV 2.56 × 2.56 × 2.56 cm (DV × LR × Rostral-Caudal). Reconstructed data were exported and visualized with “Image-J” software (National Institutes of Health). MRIs bearing iron deposits were compared with a Pteronotus parnellii brain atlas (W. O'Neill, unpublished observations) to infer their anatomical location and to increase the accuracy of future recordings in the same animal.
To determine the absolute anatomical location of recording sites, small electrolytic lesions were made following some recording sessions at several locations along the electrode track. Bats were deeply anesthetized by subcutaneous injection of a mixture of tribromoethanol and amylene hydrate (Avertin) and perfused transcardially with 4% paraformaldehyde in PBS. The brain was postfixed and cryoprotected with 30% sucrose in PBS before coronal sections (40-μm thickness) were cut on a cryostat for recovery of lesions and recording locations.
Data analysis.
Waveforms representing single unit activity were identified offline with Spike2 software using template matching or by manual separation of waveform clusters using waveform parameters, such as “spike height” and “peak time” as well as principal components. Waveforms representing single unit action potentials generally had a peak-to-peak amplitude more than three times that of the baseline activity in the recordings (Supplemental Fig. S2A) and formed discrete clusters when viewed in either two or three dimensions.
We analyzed call responses in 250-ms windows following stimulus onset. A no-stimulus interval (equal to the response window) was recorded before each presentation of the call array (or randomized block of calls) to provide a relevant record of spontaneous activity (Supplemental Fig. S2C). To identify call-evoked enhancement and suppression of the firing rate in the 250-ms poststimulus window (referred to as the “mean firing rate”), we compared both response parameters across each stimulus repetition to no-stimulus control intervals (P < 0.01, Wilcoxon rank sum test, two-tailed).
In many cases, call presentation did not evoke a significant change in the mean firing rate, but the instantaneous firing rate nonetheless appeared to vary as a function of time following stimulus onset. To capture this aspect of the response, we performed two additional statistical tests to determine the significance of instantaneous increases and sustained decreases in the spike rate following stimulus onset. First, peristimulus spike times were convolved with a Gaussian function of 2-ms SD to estimate the spike density at each poststimulus time point. Then the peak value of the spike density function in the 250 ms following stimulus onset was determined, along with the longest interval that the spike density remained below the mean of the no-stimulus condition (referred to here as “quiescence”). To assess the significance of either stimulus-evoked peaks in spike density or intervals of quiescence, null hypothesis distributions were generated for each measurement using activity recorded during the no-stimulus control intervals. Spikes from these intervals were assigned random time stamps from 0 to 250 ms before filtering with a gaussian kernel. The resulting spike density function was used to determine the peak firing rate and the longest interval of quiescence for the no-stimulus control (Supplemental Fig. S3). This procedure was repeated 10,000 times to generate a distribution representing each of the two measures taken from the randomized spontaneous activity. Call-evoked peak spike rates and intervals of quiescence were compared with these distributions whenever the neuron's spontaneous firing rate was >1 Hz.
The peak firing rate and longest interval of spike suppression (quiescence) present within the time window for the no-stimulus control were also compared with the null hypothesis distribution (P < 0.05) to identify spontaneous activity that was nonrandom (e.g., spontaneous bursting). Call responses of neurons with nonrandom spontaneous firing were excluded from these statistical comparisons. Otherwise, if the peak firing rate or longest quiescence following call presentation was greater than that expected for a random arrangement of a neuron's spontaneous spikes (P < 0.01, one-tailed), the response was considered to be significant for the neuron and call in question. Significant instantaneous peaks in the firing rate are referred to as “excitation” along with increases in the mean firing rate. Periods of quiescence persisting for a significant duration were termed “suppression” along with decreases in the mean firing rate. Excitation was sometimes preceded or followed by periods of suppression within the same “mixed” response and could coexist with an increase or decrease in the mean firing rate as well. For most analyses reported here, we pooled responses to calls presented at 65 and 75 dB SPL (unless explicitly stated) to increase our statistical power for identifying significant intervals of suppression and peak spike densities. Statistics are reported as means ± SD unless otherwise noted. We quantified the overall tendency of a neuron towards excitation or spike suppression using an index, (E − S)/(E + S), where E and S are the percentages of call stimuli evoking excitation and suppression, respectively. This was used to illustrate changes in the balance between excitation and suppression along the dorsoventral axis in the BLA. Percentages were calculated from among all call stimuli presented, including all call types and multiple pitch-shifted variants of each type.
Spike density functions over a 250-ms response window were used for a classification of call-evoked responses using cluster analysis procedures (Systat; SPSS). Mean spike density functions for each cluster were subjected to multidimensional scaling to visualize the relationship between each cluster in two-dimensional space. Factor analysis was used to estimate the number of response segments within 250 ms that contributed to the classification scheme.
To determine response-onset latency, we convolved peristimulus spike times with a causal exponential kernel having a 5-ms decay constant to form a spike density function. The response onset was marked as the first time point, following stimulus presentation, after which the spike density function exceeded the mean spike density of the no-stimulus control period by 2 SD for at least 10 ms consecutively (Kusmierek and Rauschecker 2009).
RESULTS
We describe below the basic response characteristics of BLA neurons and demonstrate the selective nature of call responses including the effect of amplitude and pitch variants of different call types within this region of the amygdala. Figure 1, B and C, shows recording locations at different depths from two different anterior-posterior levels of the BLA. After MRI, iron deposits that resulted in local field inhomogeneities were visible in the reconstructed image as dark punctate marks (Fig. 1D). Postmortem verification by histological examination of small electrolytic lesions in cross sections of the amygdala was accomplished in three separate bats (Fig. 1E).
Basic call response parameters and patterns.
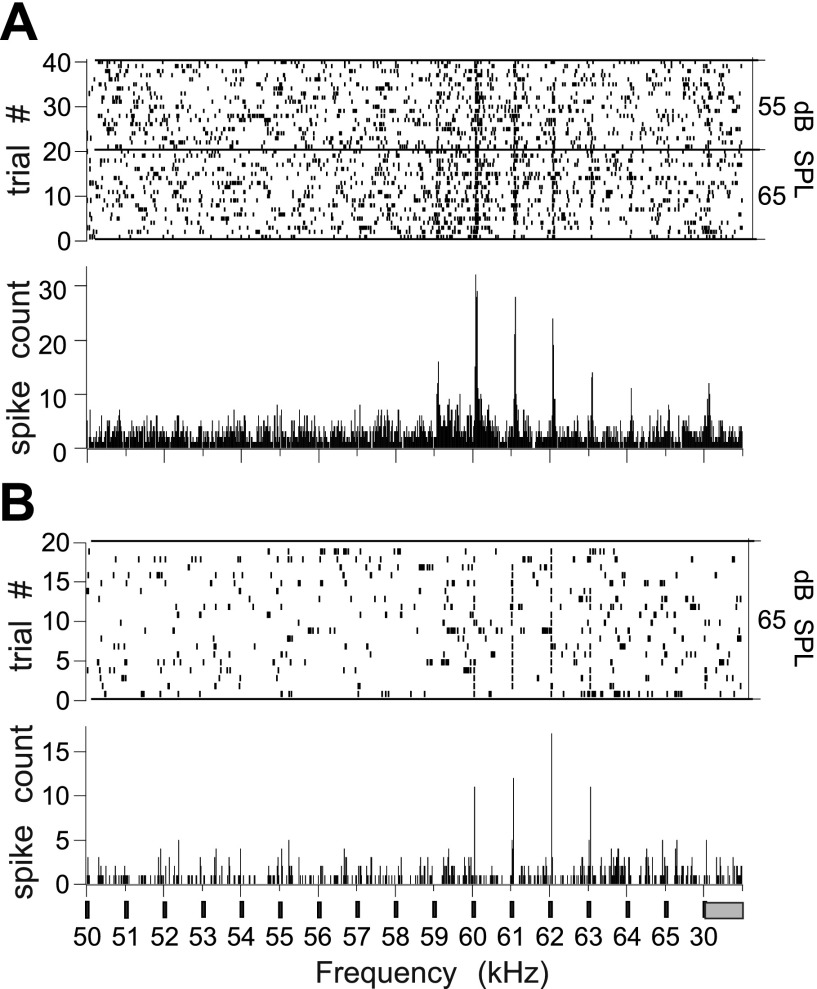
One-hundred and four single units were recorded from seven bats (4 males and 3 females). Thirty-four units were located in the left hemisphere and 70 in the right hemisphere. Tone bursts typically centered at ∼59 kHz (the bat's resting CF2 in the echolocation pulse) and stepped up and down in frequency (step size of 1 kHz) provided a preliminary characterization of a small set of neurons. Thirty-five (85%) of 41 single-units tested met statistical criteria for an excitatory response to either a single tone or a tone pair (CF1/CF2 frequencies). Nearly half (n = 19) of the units tested showed clear excitatory frequency tuning based on visual examination of peristimulus time histogram (PSTHs; examples shown in Fig. 2, A and B). Virtually all of these (96% of all tone responsive neurons) were also excited by one or more calls.
Fig. 2.
Raster plots (top) and Peristimulus time histogram (PSTH) (bottom) summed for 40 trials to show the frequency tuning to the CF2 (centered at ∼59 kHz) in a tone pair (CF1/CF2) of 2 BLA neurons, where CF is constant frequency. Neuron in A has a higher rate of spontaneous activity than the one in B. The 30-ms tone bursts were presented at a rate of 2 per second. Final stimulus was a standard tone burst at 30 kHz corresponding to the CF1 of the echolocation pulse. Bin width = 10 ms.
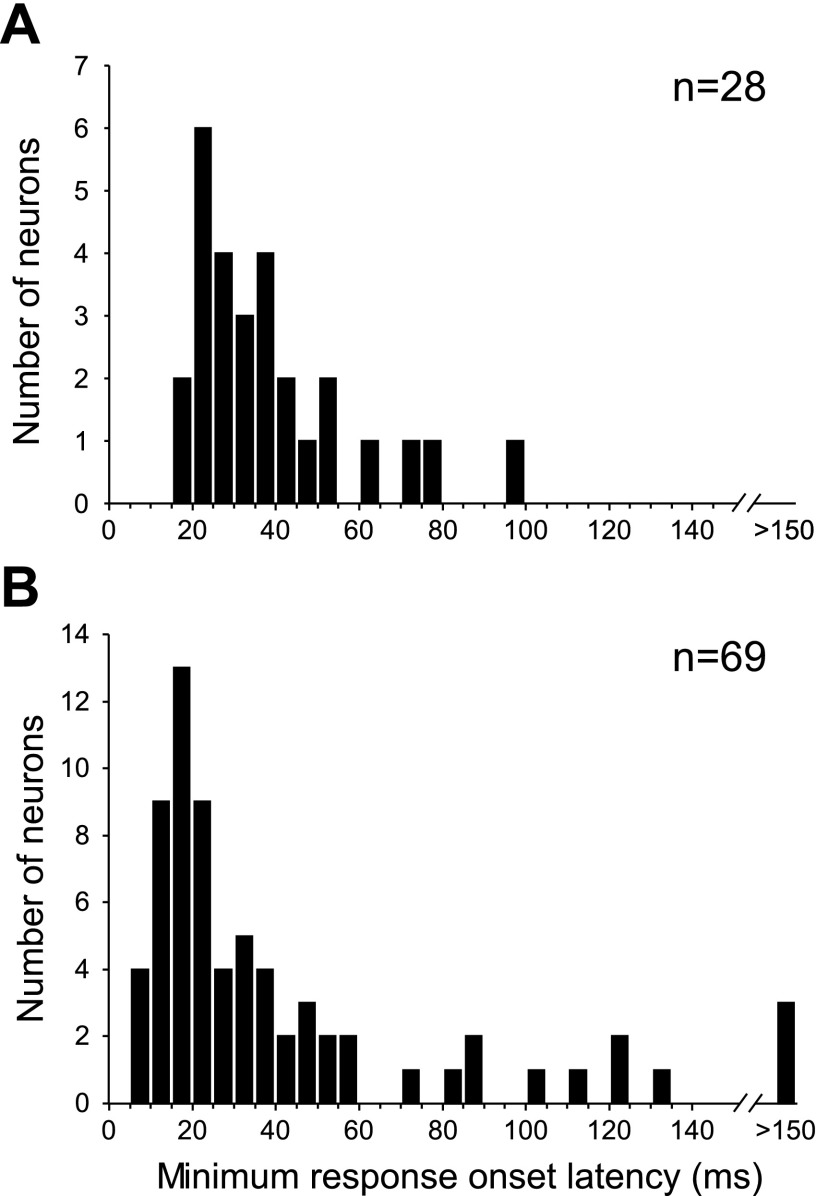
At the two highest sound intensity levels tested, nearly all (n = 100) of these units had a response to one or more calls by our criteria. Overall, calls elicited greater response magnitudes than tones in a BLA neuron that responded to both stimuli. The ratio of the best pure-tone response to the best call response was 0.82 ± 0.75 when comparing z-scores of evoked spike counts or 0.75 ± 0.56 when comparing normalized peak firing rates. With the use of equivalent numbers of tone and call stimuli (including only responses to each call at its mean pitch), the spike count z-score call-to-tone ratio was 1.41 ± 3.2 whereas that for peak firing rates was 1.05 ± 0.73. To characterize the speed of information transmission to the BLA, we calculated the minimum response onset latency across tone or call stimuli presented to each neuron. All latencies shorter than 5 ms (3 for calls, 1 for tones) were rejected, and additional latencies shorter than 10 ms were visually inspected before eliminating spurious short onsets (4 for calls, 1 for tones). The average tone response onset latency across units was 33.1 ms (Fig. 3). Across call-responsive neurons, the average call response-onset latency was 41.8 ms. A longer average latency for calls likely reflects the relative spectrotemporal complexity of the call stimuli, with neurons responding at the onset of a preferred acoustic feature(s) rather than absolute call onset.
Fig. 3.
Density histograms showing the distribution of response-onset latencies calculated for single units' excitatory responses to tones (A) and calls (B).
Ninety-three percent (93/100) of call responsive units exhibited a phase of excitation to one or more calls. A subset of 82% (82/100) of units showed significant call-evoked peak firing rates (measured from the Gaussian-convolved spike density functions) and 69% (69/100) showed significant increases in the mean firing rate (over a 250-ms duration following call onset). Seventy-six percent (76/100) of call-responsive units showed periods of call-evoked quiescence that were longer than those expected by chance and thus considered to reflect spike suppression, and 16% (16/100) of the units showed significant decreases in the mean spike rate following call onset. Thirty-three percent (33/100) of the units exhibited a mixed (excitation and suppression) response pattern to at least one call type. Table 1 summarizes some of the basic response properties of spiking in the BLA.
Table 1.
Basic response characteristics of BLA neurons as determined from spike density functions
| Measure | Mean | SD | Range |
|---|---|---|---|
| Spontaneous firing rate, Hz | 3.7 | 3.3 | 0.15–19.9 |
| Shortest excitatory response onset latency, ms | 41.8 | 40.7 | 7.4–180.8 |
| Average latency to peak firing rate, ms | 81.9 | 42.3 | 18.8–224.4 |
| Normalized best peak firing rate (SD > spontaneous firing) | 20.76 | 19.5 | 3.9–113.7 |
| Avg. magnitude of increase in mean spike rate, Hz | 6.2 | 5.4 | 1.7–45.5 |
| Avg. magnitude of decrease in mean spike rate, Hz | 4.0 | 1.6 | 2.2–8.3 |
| Excitatory phase response duration, ms | 11.4 | 6.9 | 1–31.7 |
| Inhibitory phase response duration, ms | 94.0 | 40.3 | 37–250 |
Means, SD, and ranges for call-response measurements made from basolateral amygdala (BLA) neurons. Response latencies, magnitudes, and durations were averaged across significant call responses for a given neuron before averaging each measure across neurons, thus giving each neuron an equal weighting despite differences in the number of call responses across neurons. Excitatory and inhibitory response magnitudes and durations were calculated separately. Excitatory durations reflect the longest continuous interval that the spike density function exceeded 2 SD above the spontaneous spike density. Inhibitory durations were calculated in a similar manner (see MATERIALS AND METHODS for details). Response magnitudes are expressed as the change in the mean spike rate over the whole 250-ms interval following stimulus onset and are reported for units with evoked mean firing rates greater than (n = 69) or less than (n = 16) spontaneous (P < 0.01, Wilcoxon rank sum text).
Response adaptation.
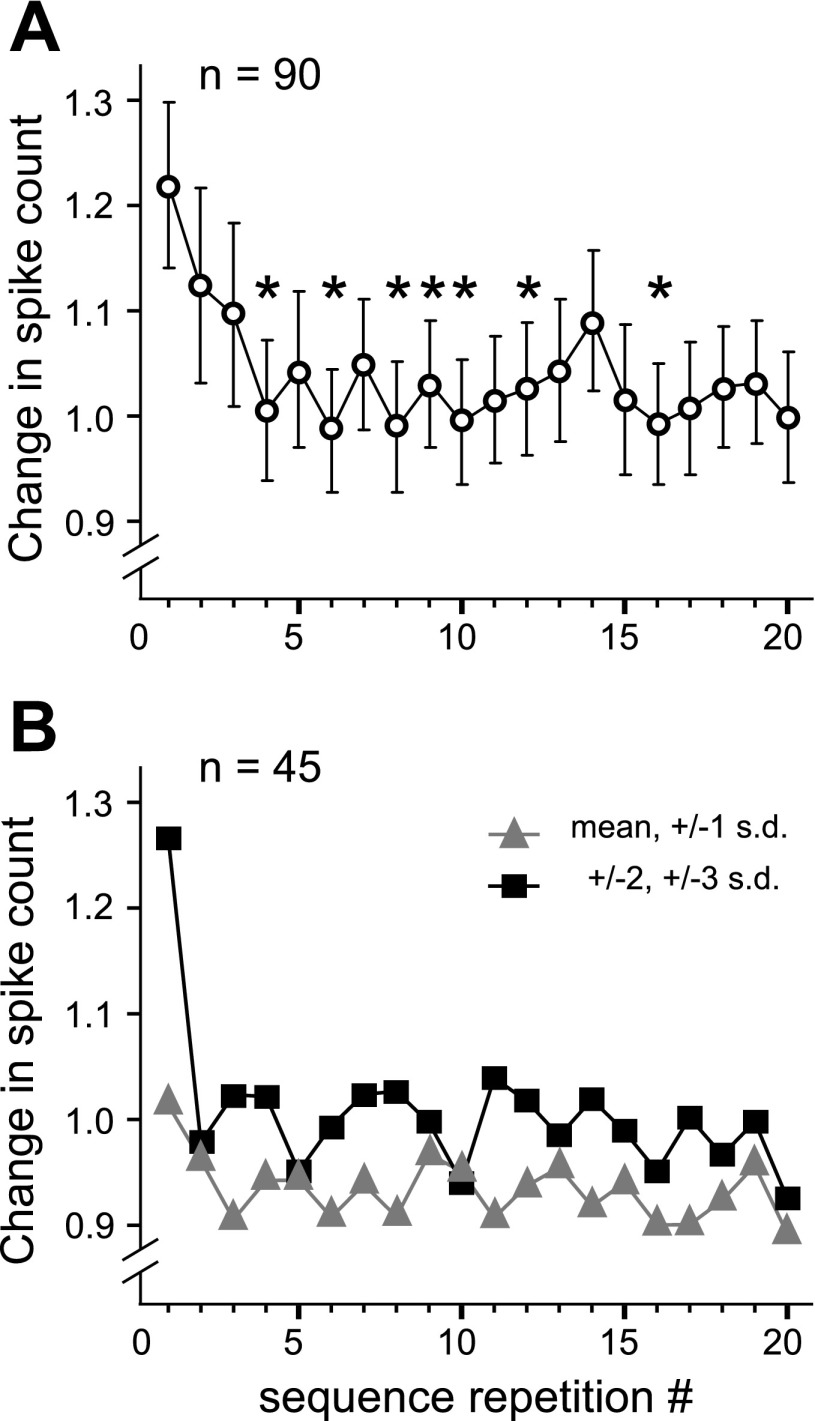
To determine whether call responses adapted over repeated presentations, we analyzed stimulus-evoked spiking and spike suppression across stimulus repetitions at the highest sound intensity for those neurons where calls were presented as an ordered set with a constant repetition rate of 1 or 2 Hz (n = 90). For each of the 20 repetitions of the stimulus sequence, a net change (from the average spontaneous level) in total spike count was calculated for each call and summed across all call types. Response suppression was accounted for by rectifying the negative counts. The mean change in spike count for all neurons is plotted in Fig. 4A. A repeated-measures ANOVA (with Greenhouse-Geisser correction) revealed an effect of trial number on the absolute response [F(6.16,547.92) = 3.6, P < 0.001]. Post hoc t-tests with Bonferroni correction showed that the response to the first sequence presentation was higher than the response to the fourth presentation and several subsequent ones (Fig. 4A, asterisks).
Fig. 4.
Adaptation of responses across call presentation trials. A: change in spike count averaged across neurons (n = 90) that were presented with repeating sequences of calls. For each sequence repetition, an average response (absolute value of evoked minus spontaneous spike count) across call types and pitches was plotted to visualize the trend in response magnitude across repetitions. *Repetitions where the response was lower than that evoked by the first call sequence (P < 0.01, one-tailed t-test). B: separate plots of response magnitudes for typical and atypical pitch variants of calls. Neurons tested with both types of calls tended to have a stronger response to atypical variants on the first sequence repetition, and a higher steady-state response, pooled across the remaining 19 repetitions.
Initial dissection of response adaptation according to the pitch of a call indicated that responses to typical pitch variants (i.e., those at the mean pitch or shifted by 1 SD above or below the mean) exhibited greater adaptation than atypical pitch variants (those shifted in pitch by either 2 or 3 SD). Average responses across repetitions of the stimulus sets from each of these two pitch categories are plotted separately in Fig. 4B. A two-way repeated measures ANOVA comparing responses across pitch category (2 levels; typical vs. atypical) and stimulus repetition number (20 levels) showed a significant interaction between the two factors [F(11.14,490.14) = 2.425, P = 0.006] and significant main effects of both trial number [F(8.66,381.02) = 4.52, P < 0.001] and pitch [F(1,44) = 5.53, P = 0.023]. For calls presented at atypical pitches, the average response to the first presentation was 27.6% higher than the average of the subsequent 19 presentations. In contrast, the first presentation of calls at common pitches was just 9.1% above the average for the remaining presentations. Paired t-tests were used to compare BLA neurons' responses between the typical and atypical call sets at each trial number. Across pitch categories, response magnitudes on the first presentation were significantly larger for atypical pitch variants than for the typical (i.e., common) pitch variants. (P = 0.002, corrected for 20 comparisons). Differences in response magnitude were only marginally significant for one of the remaining 19 presentations (trial 8, P = 0.044 corrected for 20 comparisons). Repeating the ANOVA after the data were removed from the first stimulus presentation resulted in elimination of both the main effect of stimulus repetition number [F(10.91,480.18) = 1.06, P = 0.39] and the interaction between pitch category and repetition number [F(10.86,477.70) = 1.25, P = 0.25]. The main effect of pitch category remained marginally significant [F(1,44) = 4.21, P = 0.046], with responses on successive trials with atypical pitches being higher than those with typical pitches. For a subset of neurons (n = 14), randomization of call order within a pool of typical and atypical pitch variants did not show a significant effect of trial number indicating that predictive ambiguity of the call sequence may play a role in keeping the response in successive trials at the same level as the first trial. Additional data are needed for a rigorous test of this hypothesis.
Variability of response patterns within and between call types and neurons.
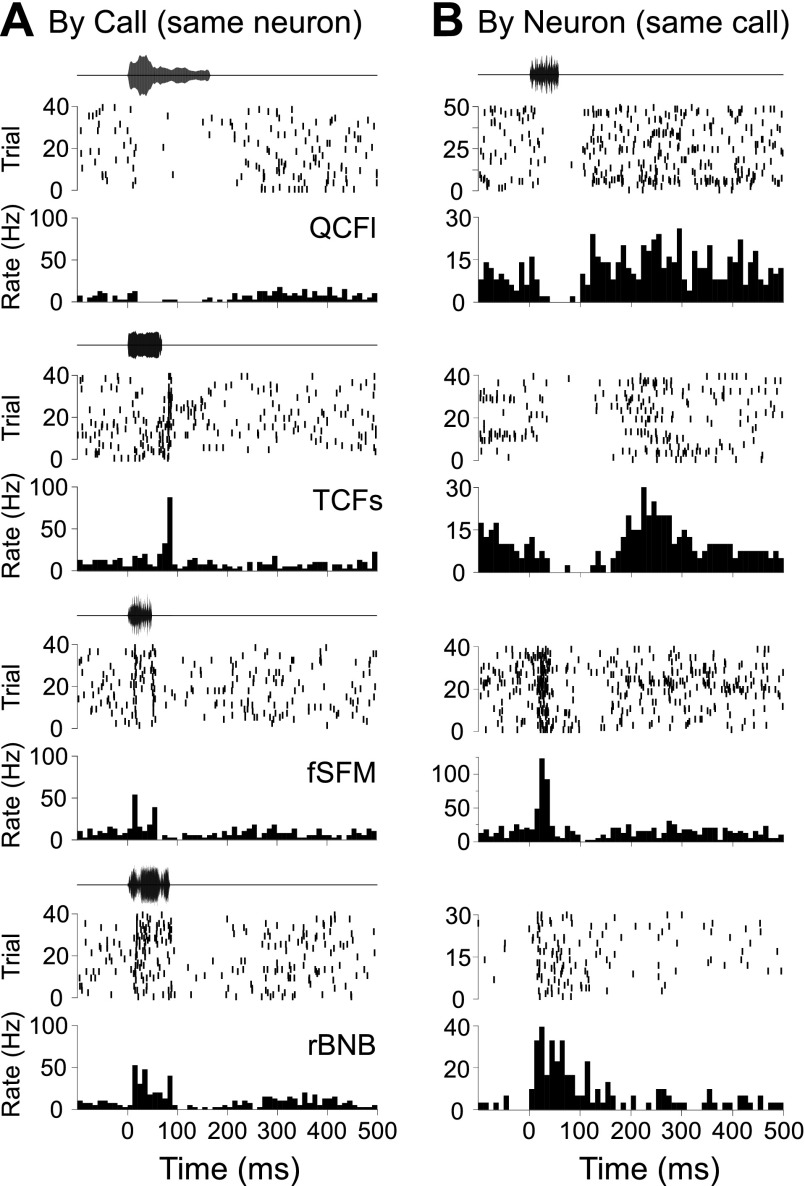
For a given neuron, temporal response patterns tended to vary with the call type presented. Figure 5A shows the response of a neuron to four different call types. This unit had a strong spiking response to the rectangular broadband noise burst (rBNB) call type, whereas spiking was suppressed by the long quasi-constant frequency call (QCFl) call type, a pattern exhibited by other units in our sample as well. Note the similarity in the response pattern to two different call types (fixed sinusoidal frequency modulation and rBNB) in the same neuron. Across neurons, some call types tended to evoke variable response patterns, while others were more consistent. Figure 5B shows a set of reponse patterns that consist of spike suppression, excitation followed by suppression, and simple tonic excitation (bottom) evoked in four different neurons by the same call type, the stretched rippled frequency modulation (sRFM). Neurons could not be easily classified into different types based on either their response to calls or their temporal response patterns. Accordingly, we focused our attention on a statistical classification of over 1,600 single unit call responses obtained from 100 units in the BLA, irrespective of neuron and of call type presented.
Fig. 5.
Examples illustrating variability of responses across BLA neurons and different call types. Amplitude envelopes of the call stimuli are illustrated above raster plots and peristimulus histograms representing stimulus-evoked action potentials. PSTH bin width is 10 ms. A: different temporal response patterns evoked in a single BLA neuron in response to 4 different calls. B: suppression, excitation, and combinatorial temporal response patterns evoked in 4 different neurons in the BLA, each in response to the stretched rippled frequency modulation (sFRM). QCFl, long quasi-constant frequency call; TCFs, true constant frequency call; fSFM, fixed sinusoidal frequency modulation; rBNB, rectangular broadband noise burst.
Classification of temporal response patterns.
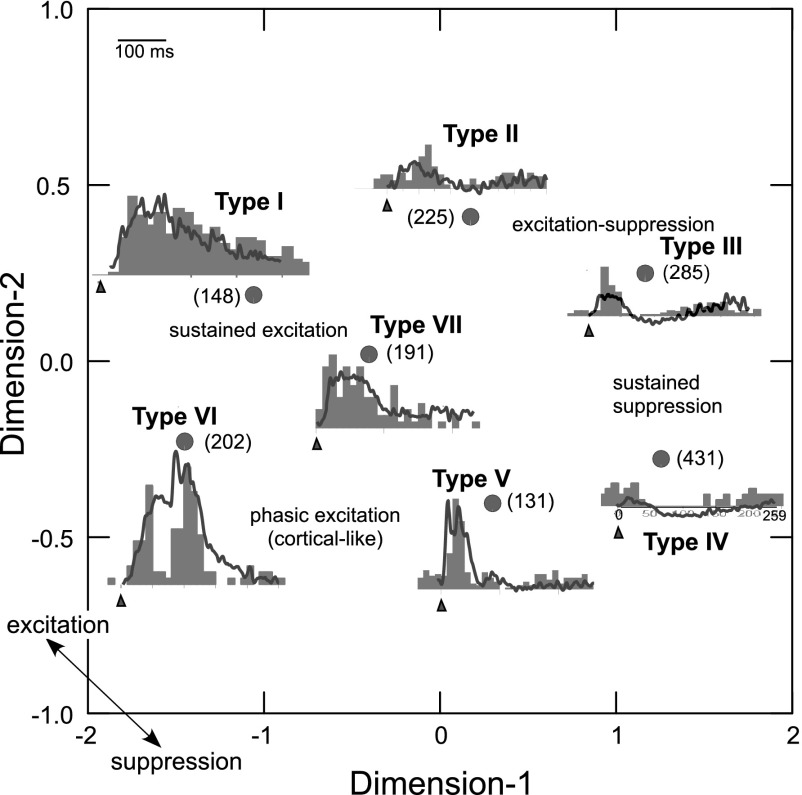
To generate a meaningful classification, we began with a visual examination of call-evoked response patterns. This revealed three possible response types based on the presence of excitation, inhibition or both. By tabulating response-onset latency and the timing and duration of modulation of the spike rate, mixed (excitation/suppression) types of responses were further divided into five types. Spike density functions generated for all responses were downsampled to 1-kHz sampling rate yielding an array of 250 time points per response. The resulting data were subjected to cluster analysis by seeding the distribution with seven clusters. Cluster identity of each response pattern was estimated using Mahalanobis distances (K-means classification procedure) to minimize within cluster variance. Stepwise method of discriminant analysis was used to test the model and yielded reasonably good prediction for group membership with 59% of original grouped cases being correctly classified.
We initiated a response-pattern analysis by pooling responses across all neurons and all calls with all call types presented at their mean value of pitch. Cluster means for each of the seven clusters representing different response types were used to compute a correlation matrix and the coefficients representing relationships between each pair of clusters were subjected to a multidimensional scaling (MDS) algorithm (Kruskal method). The MDS scheme (scaled for 2 dimensions) revealed an oval configuration where all cluster means were plotted at an optimal distance from each other (Fig. 6). In this plot, each dimension represents a composite of multiple parameters: response segments representing the level of excited vs. inhibited state of the response at each time point that show higher correlations to each other compared with those included in the orthogonal dimension. A response pattern exhibiting sustained excitation lasting > 250 ms (Type I) was present in nearly 10% of the neurons. The configuration represents decreasing levels of excitation and increasing levels of spike suppression moving from top left to bottom right. Response types were labeled from Type I to Type VI in a clockwise fashion starting with the sustained response. The response types do not necessarily reflect tight clustering of temporal response patterns, rather they represent the average of response patterns that correspond to the centroids of each cluster and inform us of the response landscape in the BLA. A descriptive label indicates the location of 4 basic patterns of excitation and/or suppression in the response space. Type II responses were largely excitatory, but the increase in mean spike rate did not last as long as for Type I responses. Types III and IV showed mainly spike suppression with a relatively low magnitude, phasic excitation at the stimulus-onset and a small excitatory rebound following spike suppression. Type V responses consist of cortical-like (Washington and Kanwal 2008), phasic excitation followed by either quiescence or mild spike suppression. The short sustained pattern in the center is labeled as Type VII and completes the circular configuration representing different types of response patterns. The two-dimensional configuration captured 99% of the parametric variation. To visualize the response pattern represented by each cluster, an exemplar PSTH resembling the mean response pattern for each cluster is superimposed at the appropriate location in the MDS scheme (Fig. 6).
Fig. 6.
A multidimensional scaling (MDS) configuration of temporally complex response patterns. Relative location of 7 cluster means of spike-density function data are indicated by filled circles. Response pattern corresponing to each data point is shown by line plots of the mean spike density function superimposed on example PSTHs (10-ms bin width) obtained in response to the presentation of different call types and from different recording locations. Axes represent composite of parameters used to quantify response patterns. One-sided arrowheads on the tilted axis show the general mapping of inhibition vs. excitation. Dashed lines with arrows indicate hypothetical streams for transitioning from sustained excitation to sustained suppression. Triangles under PSTHs indicate stimulus onset and numbers in parantheses indicated numbers of responses represented by each cluster mean.
A principle components method of factor analysis (SYSTAT software, SPSS) yielded seven factors that captured 60% of the total variance observed in the spike density functions spanning 250 ms of the response duration. Each additional factor captured <2% of the total variance. This suggested that up to seven features (response segments) contribute information useful in the classification process with a major portion of the variance being explained by the first three factors.
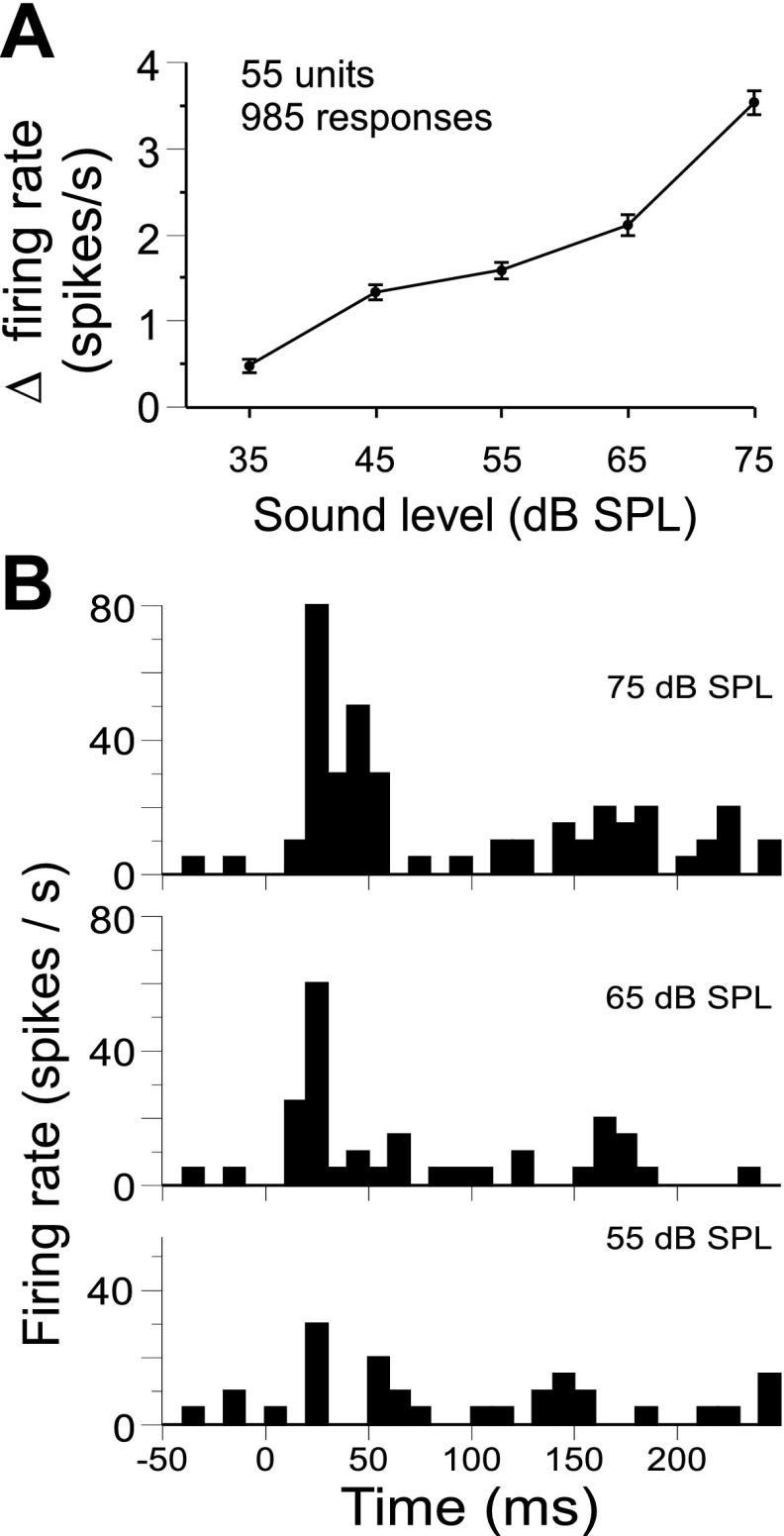
Representation of amplitude and rate-level functions.
Fifty-five units were presented with calls at three to five sound levels. A given call sequence was presented 20 times at each attenuation level, and evoked changes in the mean firing rate (i.e., evoked minus spontaneous) were quantified for each combination of call type and stimulus intensity, resulting in many rate-level functions for each neuron. Rate level functions (n = 985) were pooled across stimuli and neurons, including only those stimuli that evoked a significant excitatory response at one or more intensities. Spike count and peak firing rate were the response parameters evaluated for significance (see materials and methods for details). Firing rates were then subjected to a one-way ANOVA with stimulus intensity as the independent variable, revealing a significant effect on response strength [F(4,4532) = 67.3, P < 0.001]. We also performed an ANOVA using only a single average rate-level function per neuron (i.e., averaged across all responses of a given neuron) that produced similar results (not shown). Figure 7A shows that mean changes in firing rate evoked by calls increase monotonically with increasing stimulus amplitude. For each neuron tested at five or more sound levels (n = 32), we generated a single rate-level function by averaging across significant call responses before calculating the correlation between the mean firing rate and sound level. Eighteen of 32 (56%) units showed significant correlations between sound amplitude and changes in mean firing rate [r(3) > 0.805, P < 0.05, one-tailed], and 1 of 32 showed a significant inverse correlation. Figure 7B shows a neuron's response to the single arched frequency modulation at three different amplitude levels. Note that the response pattern (Type II) does not change dramatically across call amplitudes.
Fig. 7.
Dependence of mean firing rate on stimulus amplitude. A: changes in the mean firing rate were averaged across excitatory call responses for each sound level. On average, response strength increased with increasing sound amplitude [F(4,984) = 67.3, P < 0.001 one-way ANOVA]. B: an example of call response matching the average trend shown in A. Individual PSTHs are generated from 20 presentations of the single arched frequency modulation at each of the top 3 stimulus amplitudes as indicated.
Representation of pitch.
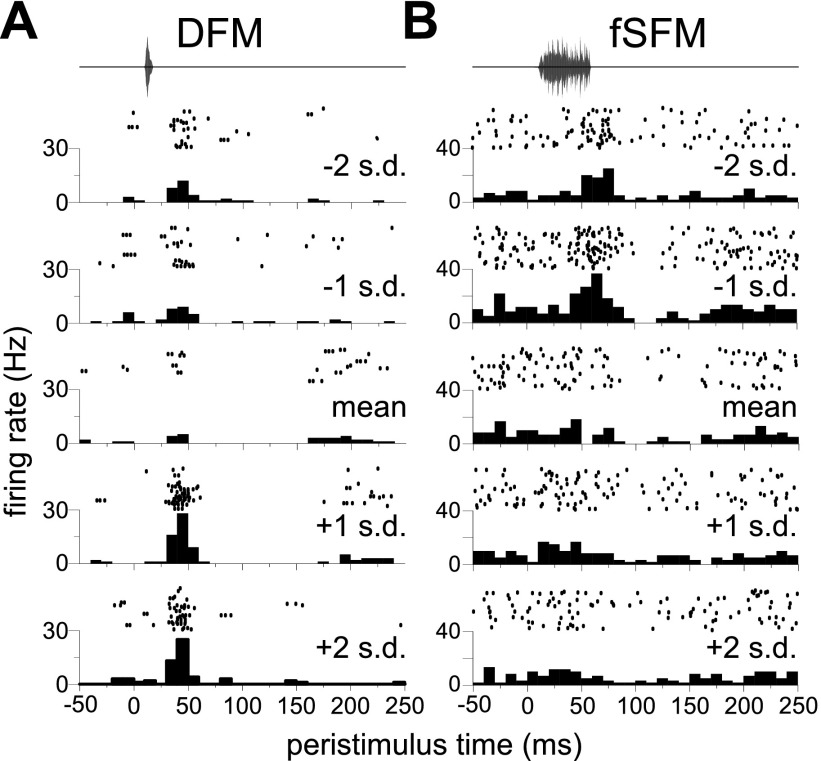
We first sought to determine how often the magnitude of a response to a given call type varied across call pitches. Sixty-nine units were presented with multiple sets of 14 simple syllabic calls at different pitches. As indicated in the description of acoustic stimuli, pitch variants were standardized for each call type, i.e., expressed in units of standard deviations relative to the mean pitch of a call type within the sample of recorded calls. Call types evoking a change in the mean firing rate (above or below spontaneous, P < 0.01, Wilcoxon rank sum test, two-tailed) at any pitch were subjected to a one-way ANOVA with call pitch as the factor (3–7 levels per response). Of the 279 such responses recorded from these 69 units, 159 (57%) showed significant variation across call pitch (P < 0.01). Figure 8, A and B, shows examples of neurons exhibiting significant variation in spike count across pitches for two different call types.
Fig. 8.
Pitch dependance of firing rate of two example neurons in response to 2 different call types, the downward frequency modulation (DFM; A), and the fSFM (B). Call pitch (expressed as SD above or below the mean pitch of the exemplars for the call type) is indicated above each PSTH.
Of those units analyzed above (i.e., the 69 presented with multiple pitches) 50 had a response to more than one call. For these neurons, we performed a two-way ANOVA with firing rate as the dependent variable and with call type and call pitch as factors. Seventy-two percent (36/50) of these units showed a significant interaction between call type and call pitch, indicating that for these neurons the effect of shifting the pitch depended on the call type in question. Accordingly, the preferred (best) pitch varied with call type. A small minority of units (8/69) preferred the same pitch across all call types (significant main effect of pitch and no interaction between pitch and call type). These could be considered as pitch-selective neurons.
Representation and selectivity for call types.
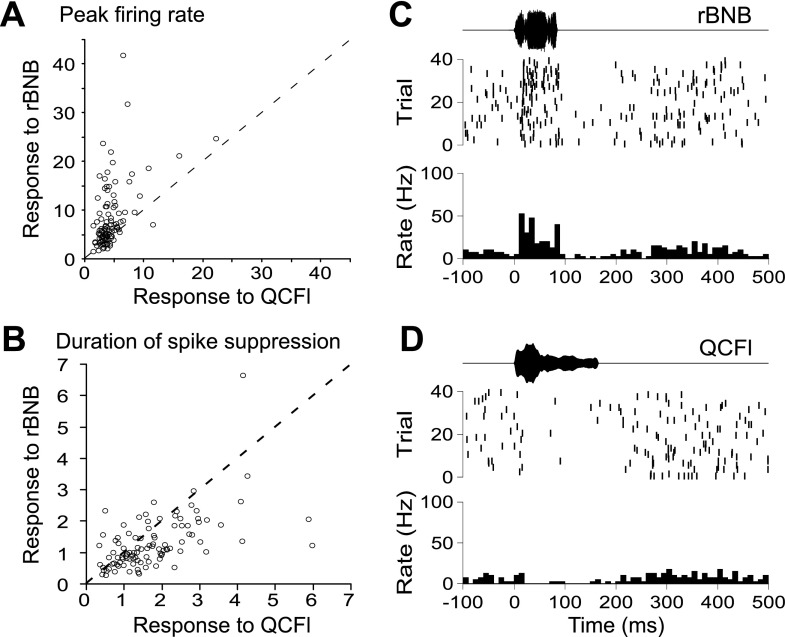
Unlike the AC in this species (Ohlemiller et al. 1996; Washington and Kanwal 2008), a substantial percentage of BLA call responses consisted of either transient or sustained spike suppression. We sought to determine whether call types differed in the frequency with which they elicited excitation vs. suppression. We were especially interested in examining the response to two call types, which are distinct from a behavioral perspective. The QCFl is used in affiliative social interactions, and the rBNB is used before and during aggressive interactions (Clement et al. 2006). Figure 9A shows a scatterplot comparing each neuron's response to the rBNB vs. its response to the QCFl, in terms of the peak firing rate (expressed as a z-score relative to spontaneous activity). Figure 9B shows the same for the duration of spike suppression (expressed as a multiple of the longest spontaneous quiescence; see materials and methods and Supplemental Fig. S3 for details). Paired t-tests confirmed the tendency for the rBNB to evoke higher peak firing rates than the QCFl (t104 = 6.8, P < 0.0001), resulting in the majority of data points (representing individual neurons) lying above the diagonal in Fig. 9A. Paired t-tests also verified that periods of quiescence were longer in response to the QCFl than for the rBNB (t104 = 3.85, P = 0.0002). Note the scattering of points below the diagonal in Fig. 9B. Figure 9C provides an example raster plot and PSTH from a neuron with a robust increase in spike firing in response to the rBNB, and Fig. 9D shows that the QCFl evokes a robust decrease in spiking in the same neuron.
Fig. 9.
Dichotomy of responses evoked by an aggressive call vs. an affiliative call in BLA neurons (the rBNB and QCFl, respectively). A: peak firing rates evoked by the aggressive rBNB call (y-axis) and the affiliative QCFl call (x-axis), averaged across pitches, are plotted against one another for each of the sampled neurons. Firing rates are expressed here as standard deviations above and below the mean spontaneous firing rate. The rBNB evoked a higher peak rate than the QCFl in a majority of cells. B: intervals of spike suppression evoked by the rBNB and QCFl were expressed as a proportion of the longest quiescence measured from the spontaneous activity, and plotted for each unit. The affiliative QCFl tended to evoke longer suppressions than the rBNB. C and D: raster plots and PSTHs from an example BLA neuron showing increased spiking in response to the rBNB (C) and decreased spiking in response to the QCFl (D).
To broadly characterize the preference of BLA neurons for particular call types, we counted the number of excitations and spike suppressions evoked by each call type. The rBNB and true constant frequency (TCFl) call types evoked mainly excitatory activity, with 139 excitatory responses vs. 45 suppressions for the rBNB, and 116 vs. 36 for the TCFl. In contrast, the short quasi constant frequency (QCFs) and QCFl suppressed spiking more than other calls, with 24 instances of excitation vs. 66 of suppression for QCFs, and 27 instances of excitation vs. 82 of suppression for QCFl. Three frequency modulated calls, the bUFM, sRFM, and hDFM, did not preferentially evoke facilitation or suppression in our sample but accounted for 44% of evoked alternations between excitation and suppression (i.e., “mixed” responses). The narrowband noise burst (noted to be a physiological sound akin to a cough or sneeze; Clement et al. 2006) evoked the smallest number of significant responses (excitatory or inhibitory) among all call types tested.
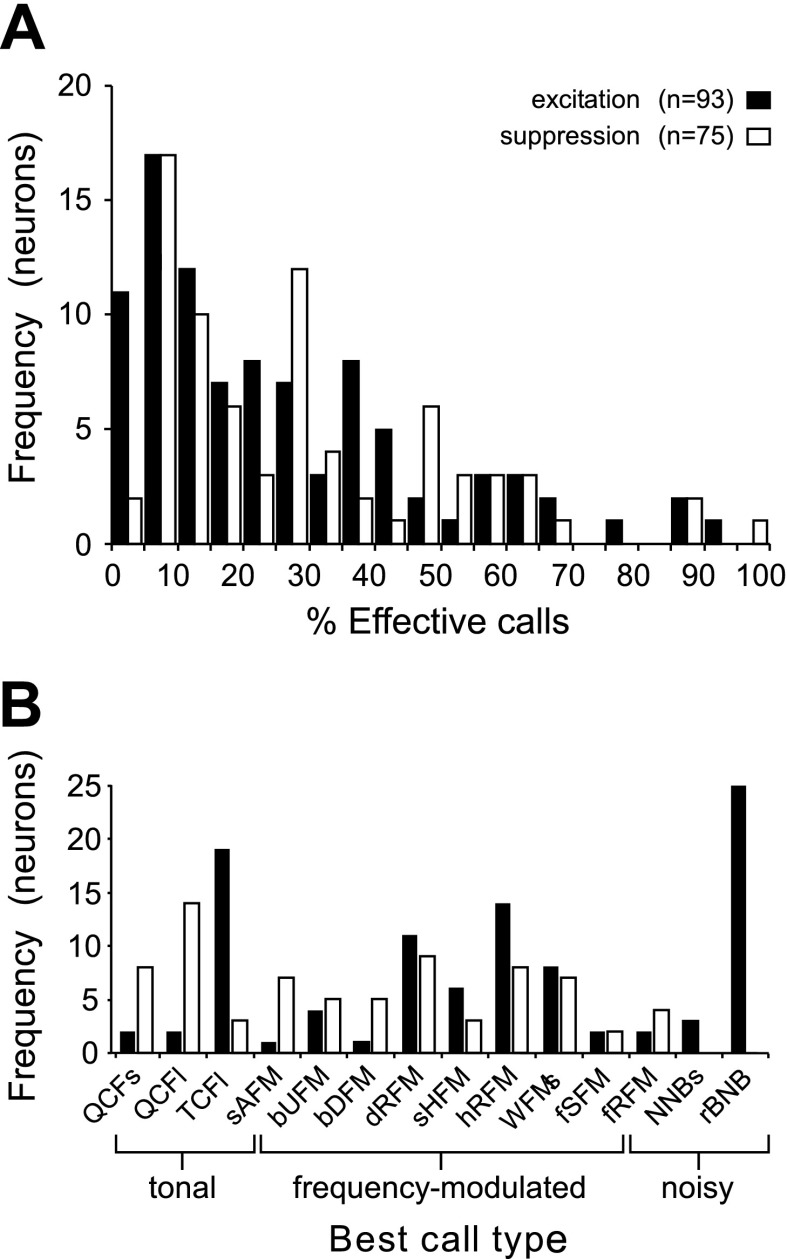
To provide a measure of call selectivity, we quantified the percentage of calls eliciting significant excitation and those eliciting significant suppression in each neuron. Distributions of these percentages are provided in Fig. 10A. Of the units with a significant excitatory response, 85% responded to fewer than half of the calls presented, and on average these units had an excitatory response to 26% of the stimuli presented. For neurons exhibiting significant call-evoked spike suppression, 79% were inhibited by fewer than half of the calls, and on average spiking was suppressed in response to 29% of calls. To characterize responses to the most common call types, we separately calculated the percentage of effective stimuli for each neuron from among the 14 call types at their mean pitch. Seventy-five units had an excitatory response to one or more calls in the mean call set and of these 80% (60/75) were responsive to fewer than half of the 14 calls. Of 58 units with an inhibitory response, 67% responded to fewer than half of the calls. Thus neurons generally responded to fewer than half (and frequently fewer than one quarter) of the call types tested at their mean pitch.
Fig. 10.
Call selectivity and preference across neurons. A: frequency histogram showing distribution of call driven excitation and suppression in BLA neurons. Position on the x-axis reflects the percentage of the total number of stimuli that evoked a response in a neuron, i.e., a simple selectivity value. A majority of neurons responded to <25% of the calls. B: bar graph indicating the number of neurons for which a given call type evoked the strongest excitatory (filled bars) or inhibitory (open bars) response. Ordering of calls along the x-axis reflects the similarity of spectrotemporal features between call types. MDS procedure was used to sequence call types in one dimension and determined the presentation order shown here (Kanwal et al. 1994).
Finally, we calculated the frequency with which each call type could be labeled as the “best call” for a neuron. The best call was determined for each neuron across excitation and/or suppression of spiking activity by identifying the maximum and minimum mean firing rates, respectively, evoked by any call type that produced a significant response (averaging across pitch variants for each call type). The rBNB call evoked the best excitatory response in the largest number of neurons. The TCFl call type was a close second (Fig. 10B, filled bars). Together, these two call types evoked the highest mean firing rate in 44% of neurons and the strongest spike suppression in only 4% of neurons (3 of 75; Fig. 10B, unfilled bars). The affiliative QCFl call type yielded the largest decrease in the mean spike rate (best suppression) in 19% of neurons and evoked the highest increase in only 2% of the neurons.
Spatial organization of call responsive neurons.
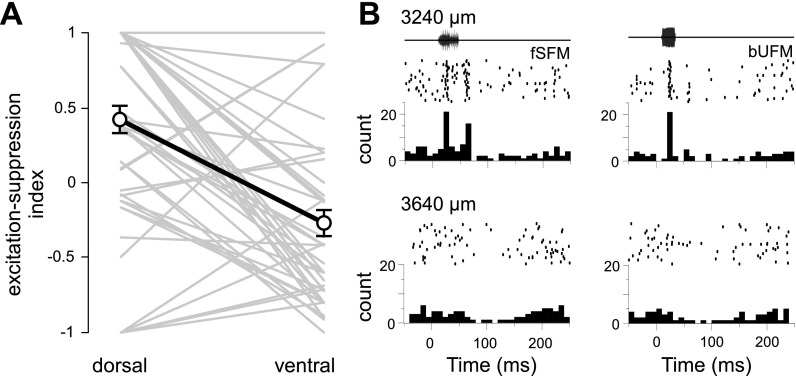
Locations of 55 single units recorded from 48 different sites were reconstructed from the stereotaxic coordinates of lesions recovered from 3 brains. Using responses to calls presented at 65–75 dB SPL, we determined the percentage of stimuli evoking an excitatory response, the percentage evoking spike suppression, or the percentage evoking either type of response. We calculated the correlation of each of these selectivity measures with the dorsoventral, mediolateral, or rostrocaudal location of each unit. For each direction, the magnitude of the correlation coefficients did not exceed 0.17, indicating the absence of a strong relationship between call selectivity of units' responses at relatively high stimulus amplitudes (>60 dB SPL) and their absolute anatomical location. As a further test, we examined cases where two or more neurons were recorded at different dorsoventral locations along the same electrode penetration (instances where >2 neurons were recorded within a penetration resulted in multiple pairs). Each of 51 pairs of neurons was analyzed for differences in response properties between the relatively dorsal or ventral neuron, eliminating much of the variability between penetrations. A paired samples t-test showed that a larger number of calls evoked excitatory responses in the dorsal group of neurons than in the ventral group (23 vs. 14%, P = 0.009, two-tailed). In addition, a greater percentage of call stimuli evoked spike suppression in ventrally than dorsally located neurons (dorsal 11%, and ventral 33%; P < 0.001, paired t-test, two-tailed). Our sample of ventral neurons showed a higher average level of spontaneous activity (3.1 vs. 4.9 Hz). To confirm that the higher spontaneous activity in ventral neurons did not result in a bias toward detection of call-evoked spike suppression in this subsample, the t-test was repeated after matching the spontaneous firing rate across the two groups (P = 0.56) by eliminating five ventrally located neurons that had the highest spontaneous firing rates. This caused a slight increase in the statistical significance of the observed difference. Figure 11 illustrates the trend towards fewer excitations and more suppression at relatively ventral locations, using an index that takes into account the relative percentages of stimuli evoking excitation or suppression for each neuron (see materials and methods for details).
Fig. 11.
Ratio of call-evoked suppression to evoked facilitation increases in a dorsal-to-ventral direction. A: a facilitation-to-suppression index (see materials and methods) was used to represent the relative number of the 2 responses types recorded from pairs of neurons at more dorsal or ventral locations along the same electrode penetration (n = 51 pairs). Lines joining individual pairs of neurons are drawn in gray, and means for each group are joined in black (error bars represent SE). B: amplitude envelops of calls, spike raster plots, and PSTHs representing a dorsal (top) and ventral (bottom) neuron recorded along the same electrode track. Vertical depth from the cortical surface is indicated above each row.
DISCUSSION
The amygdala is important for associating sensory stimuli with aversive and rewarding experiences (Davis and Whalen 2001) and plays a critical role in facilitating adaptive interactions with conspecifics (Sajdyk and Shekhar 1997; Truitt et al. 2009) as well as the environment (Killcross et al. 1997). Activity of many neurons within the amygdala is positively correlated with social interaction (Katayama et al. 2009). Species-specific calls constitute relevant and reliable signals to induce rapid changes in the motivational state and behavior of the receiver. Therefore, we hypothesized the presence of a robust representation of species-specific calls within the amygdala. Our single unit data provided a rigorous test of this hypothesis and the most detailed electrophysiological characterization of the representation of species-specific social calls in the amygdala thus far. We compare our findings with representation of sensory stimuli associated with affective states within the amygdala in other species, including humans, and explain how our data expand an understanding of information processing within the amygdala.
Response adaptation.
LeDoux and colleagues examined auditory response properties of amygdala neurons in anesthetized and awake behaving rats (Bordi and LeDoux 1992; Bordi et al. 1993). In these studies, they reported habituating (or adaptive) and nonhabituating responses to tones, frequency modulations, and noise bursts from the dorsal and ventral divisions of the LN and medial portions of the central nucleus of the amygdala. Fifty-nine percent of the responses they recorded from the LN habituated. Studies in the monkey (Nishijo et al. 1988a) indicate that this behavior might represent a subpopulation of neurons that initially respond to novel stimuli. In both the awake and anesthetized preparations, a majority of neurons in the LN showed adaptation. In our experiments, we did not attempt to record from neurons that showed strong adaptation, we also did not prolong the interstimulus interval to allow neurons to recover from possible adaptation. Despite reporting results from only those neurons with call responses that were relatively consistent across trials, we did observe a tendency for neurons to respond more robustly to the first presentation of the call set, compared with all of the successive presentations of the same call set (Fig. 4A). This effect was especially pronounced when calls were presented at atypical rather than typical pitches (Fig. 4B). BLA neurons may show strong, but transient, excitatory responses to atypical pitch variants of calls by virtue of their novelty. Studies in awake macaque monkeys (Nishijo et al. 1988a) support the notion that the small, initial adaptive phase represents an attentional component in the overall call response. The data also suggest an effect of salience that can last for several seconds and across multiple call types presented as a set in each trial (Fig. 4A).
Pure tone responses of some neurons in the amygdala are sensitive to short-term conditioning (Repa et al. 2001). Synaptic plasticity in the amygdala may, however, persist across longer time scales (Anglada-Figueroa and Quirk 2005; Schroeder and Shinnick-Gallagher 2005). Our data on adaptation (Fig. 4) suggest that responses of BLA neurons to calls are more resistant to modification than their responses to tones. This is consistent with the idea that calls are expected to have extensive histories of reinforcement in natural contexts during development.
Call-evoked spike suppression.
Spike suppression was a prominent feature of many call responses in the BLA, in contrast to the predominantly excitatory responses recorded from the AC in the same species (Medvedev and Kanwal 2004). We found that all call types evoked spike suppression by our criteria in at least one instance, although the QCFl, QCFs, and (2 tonal calls, and a frequency-modulated call, respectively) evoked the strongest suppression in the largest number of cases. Some call responses were characterized by fast alternations between periods of facilitated spiking and periods of quiescence. The most common of these “mixed” excitatory-inhibitory responses was the stimulus-locked firing of one or two spikes, followed by a silent interval of ∼50 ms (response Type III). For many multipeaked excitatory responses or mixed excitatory-inhibitory responses, positive and negative changes in spiking probability may be driven by specific stimulus features like stimulus onset and offset, discrete amplitude modulations, spectral arches, and/or linear frequency modulations. The rBNB call type rarely elicited suppression but was prominent in producing sustained excitation (cluster for response Type I in Fig. 6).
Our demonstration of call-evoked suppression underscores the potential of inhibitory control over the output of BLA neurons for species-specific call responses. This finding is in agreement with previous reports demonstrating the inhibitory influence of afferents from the medial prefrontal cortex (Grace and Rosenkranz 2002) and various basal forebrain structures (Mello et al. 1992), and mixed excitatory and inhibitory influences of afferents from the perirhinal and entorhinal cortices (Lang and Pare 1997), hippocampal formation, and medial geniculate body (Mello et al. 1992). Some of this inhibition may be mediated via intercalated cells (Marowsky et al. 2005), which surround the BLA in addition to inhibitory interneurons within the BLA (Szinyei et al. 2000).
Temporal response structure and response organization within the BLA.
The MDS analysis revealed the presence of multiple response types within the BLA. Unlike the Doppler-shifted CF processing (DSCF) area of the mustached bat primary AC, where neurons tend to respond with very phasic excitatory peaks to most or all of their effective stimuli, temporal patterns of spiking in the BLA varied considerably between the different call types generating responses. To understand how these temporal dynamics emerge in BLA neurons from their morphologies and patterns of local and extrinsic connectivity will require future experiments that include stimulation, recording, and intracellular labeling of BLA neurons. For example, response complexity may be based on complex networks of inhibition and excitation within the LN and on reciprocal connectivity between the amygdala and auditory fields in the frontal cortex (McDonald et al. 1996). A frontal auditory field has already been described in mustached bats (Kobler et al. 1987), and many neurons here are known to produce long duration sustained excitation (Type I responses) to different call types (Kanwal et al. 2000). Further studies dissecting different call types to examine the role of predominant components, e.g., different FMs, and their influence on temporal response patterns, can provide a deeper insight into the representation of calls vs. call features within the BLA.
Our data also show that call-evoked spike suppression was stronger in more ventral regions of the BLA (see Fig. 11). One potential explanation for this finding is that the intra-amygdala flow of activity from the lateral to the basal amygdala (Pitkanen et al. 1997) might result in feed-forward inhibition at ventral locations via intercalated cells (Pare and Smith 1993). As noted above, the more ventral regions of the BLA may also receive high density input from sources outside the amygdala that are known to exert an inhibitory influence on BLA neurons (Sah et al. 2003) or that synapse selectively onto GABAergic interneurons. Our single unit data support the observation that not all neurons within the LN and BN of the amygdala are driven similarly by direct excitatory sensory inputs to the amygdala (Li et al. 1996).
Amplitude and pitch selectivity.
The majority of BLA neurons were found to be largely unresponsive to calls presented at low amplitude (<50 dB SPL). This is in sharp contrast to the dynamic response range exhibited by most cortical neurons, especially for stimulus amplitude. For example, most neurons within the DSCF area respond over a range of ∼70 dB SPL and can have thresholds <0 dB SPL (Kanwal et al. 1999). Specialized neurons within the DSCF area exhibit nonmonotonic rate-level functions and also show level tolerance in that the width of frequency tuning is unaffected by amplitude levels (Kanwal et al. 1999; Suga and Manabe 1982). Less specialized neurons, such as those in A1a and A1p, show monotonic rate-level functions and exhibit broader tuning for CF tones presented at high amplitudes compared with those at successively lower amplitudes (Asanuma et al. 1983; Peng and Kanwal 2001). In comparison, BLA neurons almost always responded to calls presented at relatively high intensities (> 50 dB SPL) and frequently ∼70 dB SPL. Call responses to very high intensities (>80 dB SPL) were not obtained to constrain nonspecific activity from being averaged with specific responses when determining call selectivity across a narrow range of amplitude levels. A qualitative change in the response pattern (excitation vs. inhibition) with amplitude level was not observed (see Fig. 7).
Of calls that were presented at several pitches, 57% evoked firing rates that varied significantly with call pitch (as illustrated in Fig. 8). Clearly call-pitch information is available in the activity of many BLA neurons, which likely represents one important dimension of the motivational significance or meaning of a call type embedded within its acoustic structure. It may also depend on the context in which a particular call is emitted (Snowdon 1982). Since many simple syllabic calls consist of relatively simple spectrotemporal elements, the patterns themselves together with their parameters, e.g., the slope of an upward vs. downward FM, the rate of an SFM, and the bandwidth of a noise burst may convey meaningful information within a call (Morton 1982, 1977) even when other important components of the social context are absent. Activity within the BLA was recently shown to be sensitive to fear-conditioned discrimination of the direction of an FM (Ma et al. 2010). Similarly, the ratio of harmonics within a CF call type may also represent a meaningful feature of a call type. This would be analogous to speech sound perception, where the ratio between two particular formants (e.g., F1 and F2) is largely specific to the intended vowel but invariant to the pitch of the speakers' voice (Peterson and Barney 1952).
Conspecific identity is also an important component of the social context and determinant of what social behaviors are appropriate during social interactions with specific individuals, especially at a distance where olfaction cannot play an important role. Since call pitch can be an important cue indicating the identity of the call emitter, BLA neurons may play a role in identifying individuals within a colony by the pitch of their calls and aid in an individual-directed response during social interactions. This idea is supported by a recent study demonstrating that mouse-eared bats (Yovel et al. 2009) can be trained to reliably discriminate conspecifics using a single echolocation pulse. In other species that rely more on vision, amygdala neurons are known to select for the identity of conspecific faces (Gothard et al. 2007; Kuraoka and Nakamura 2006).
Finally, it is important to note that magnocellular neurons in BN project directly to the inferior colliculus and may play an important role in the gating of auditory inputs at early stages of auditory processing (Marsh et al. 2002). The BLA is critically situated to influence both the control and perception of vocalizations and in the case of humans its engagement may be critical for discerning verbal content, e.g., by cuing the listener to variations in prosody (Kriegstein and Parnavelas 2003) via this pathway. Amplitude and/or pitch selective neurons in the BLA may mediate this type of gating.
Selectivity and preference for call type.
The responses of BLA neurons represent the diverse reinforcement histories and social functions of different call types. Thorough analyses of single unit auditory activity in the amygdala are few (Bordi and LeDoux 1992; Bordi et al. 1993) as are those examining responses to species-specific vocalizations (Kuraoka and Nakamura 2007). In our study, BLA neurons showed varying degrees of selectivity for the 14 call types presented. Most neurons responded to <25% of the calls irrespective of which pitch variant was used, but the effective call types varied dramatically from neuron to neuron.
BLA neurons were excited by the aggressive (rBNB) call (Fig. 9). These findings are in agreement with the established role of BLA in the encoding and expression of fear. The TCFl evoked a strong response in nearly as many neurons as the rBNB. The TCFl is used during aggressive interactions, although it is not used as a prelude to aggression (or threat; Clement et al. 2006). This suggests that a fearful/submissive individual who is the target of the aggressor emits the TCFl call type. Work by Morton (Morton 1977) suggests that calls matching the spectrotemporal structure of the TCFl are often used in mammalian species to express submission during aggressive displays or attacks by dominant conspecifics. Amygdala responses to conspecific expressions of fear, similar to responses to the TCFl reported here, have also been shown in humans (Scott et al. 1997; Whalen et al. 2004) and macaques (Gothard et al. 2007; Kuraoka and Nakamura 2006, 2007). Gil da Costa et al. (2004) used PET imaging in macaques to show that screams (submissive fearful calls) elicited more activation in the amygdala than coos (affiliative calls) and nonbiological sounds. The study by Kuraoka and Nakamura (2007) also confirmed this finding in single units and also demonstrated strong responses to aggressive grunts. In mustached bats, although the TCFl and rBNB are both arousing and associated with aversive social interactions, their effects on BLA neurons are not identical. The TCFl tended to evoke excitatory responses that were more phasic than did the rBNB. This may result in differential excitation of separate efferent pathways involved in diverse behavioral responses to calls.
We specifically examined responses of amygdala neurons to those call types that are associated with either aggression, fear, or affiliation and evoked the strongest excitatory (rBNB) or inhibitory (QCFl) responses in our sample of neurons (Fig. 9). Not all neurons responded to at least one of these two calls, which together represent a small minority of the entire call repertoire. Interestingly, fearful (TCFI) and aggressive vocalizations seldom evoked strong spike suppression, while the affiliative QCFl call, with an opposing behavioral function, showed the opposite trend in its influence on BLA neurons. This result suggests that call-evoked spike suppression might enhance the stimulus specificity of cue-elicited defensive and fearful behaviors. The QCFl call is emitted with high frequency between males that roost together peacefully in the absence of territorial aggression (Clement et al. 2006). Other studies also support the idea that the stimulus specificity of cue-elicited fear may depend on inhibition in the amygdala (Quirk and Mueller 2008; Shaban et al. 2006).
In the mustached bat, the social functions of other call types are less well characterized. Some calls may be used in association with more than one behavior or with behaviors that are not fully described in this species. In other bat species, several social vocalizations are known to mediate approach behavior. These include the sounds of other bats foraging, pup isolation calls (Schmidt-French et al. 2006), and vocal displays (e.g., “songs”) of males (Fenton 1985). Responses of BLA neurons to the remainder of the communication sounds in our stimulus set may reflect their participation in a diverse set of efferent pathways, which may include appetitive behavior and anticipation of reward (Paton et al. 2006; Schoenbaum et al. 1999; Sugase-Miyamoto and Richmond 2005; Tye and Janak 2007). The BLA is known to play a role in the formation of stimulus-reward associations (Cador et al. 1989; Nishijo et al. 1988a,b). Further investigations are required to determine how individual BLA neurons, which are selective for different sets of calls, contribute to selection of specific autonomic output and response selection at the behavioral level. Additional studies are necessary to determine how call selectivity is shaped by contextual influences. In restrained macaques, affect communicated through facial expressions enhanced the response of BLA neurons to concurrent (and congruent) vocalizations (Kuraoka and Nakamura 2007). In addition, social context likely exerts a strong influence on call selectivity of these neurons.
Neural coding of affect.
The BLA is important in mediating goal-directed behavior that is driven by appetitive and aversive conditioned associations (Cador et al. 1989; Killcross et al. 1997; Paton et al. 2006) and in updating the value of conditioned reinforcers (Schoenbaum et al. 1999; Wellman et al. 2005). Single unit recordings in nonhuman primates show that neurons within the amygdala respond selectively to the affective content and conspecific identity conveyed in images of facial expressions (Gothard et al. 2007), which may serve as a mechanism for appropriate response selection. Functional MRI studies in humans have shown that perception of laughing and crying elicits a BOLD response in the human amygdala (Sander and Scheich 2001). A PET study in macaque monkeys demonstrated amygdala activation in response to two types of conspecific vocalizations (Gil-da-Costa et al. 2004). Another study in the same species (Kuraoka and Nakamura 2007) showed that only a small minority of units (one unit in their sample) responded to the auditory stimulus alone (i.e., others responded to auditory, visual, or audiovisual stimuli) but that 51% of the visual responses were facilitated by simultaneous auditory stimuli. In bats, audiovocal communication plays a primary role during social interactions and is especially the case in insectivorous bats, where vision is relatively poor and audition is a necessary means of communication in the darkness of caves or the night sky. Therefore, finding an extensive and robust representation of social calls is consistent with their behavioral importance.
Abnormalities in the functioning of the amygdala are strongly implicated in the development of communication disorders, e.g., stuttering, autism spectrum disorders, and others that have not been systematically studied from an auditory perspective. Our results help to establish the role of the amygdala in the perception of auditory expression of emotion and possibly conspecific identity and provide the first detailed investigation of single unit activity in response to species-specific communication calls. Essentially, the amygdala presents a route by which social calls can selectively influence emotion, motivation, and behavior, including vocal output. How the auditory activity within neural circuits in the BLA is translated into a context for vocal output is not yet known.
GRANTS
This research was supported in part by the National Institute for Deafness and other Communication Disorders Grants F31-DC-01009 and R21-DC-02054 (to R. T. Naumann and J. S. Kanwal, respectively) and by intramural funds kindly provided by Georgetown University Medical Center (to J. S. Kanwal).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Ministry of Agriculture, Land and Marine Resources in Trinidad for permitting us to export mustached bats and F. Muradali who assisted with the collection and exportation procedures. Special thanks to Dr. Stanley Fricke for sharing expertise in small animal MRI data acquisition.
REFERENCES
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurol 25: 9680–9685, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex 7: 157–165, 1997. [DOI] [PubMed] [Google Scholar]
- Asanuma A, Wong D, Suga N. Frequency and amplitude representations in anterior primary auditory-cortex of the mustached bat. J Neurophysiol 50: 1182–1196, 1983. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schmidt S. Affect cues in vocalizations of the bat, Megaderma lyra, during agonistic interactions. J Acoust Soc Am 124: 598–608, 2008. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Boysen ST. Specificity of the cardiac response to conspecific vocalizations in chimpanzees. Behav Neurosci 103: 235–245, 1989. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Yellow-bellied marmots discriminate between the alarm calls of individuals and are more responsive to calls from juveniles. Anim Behav 68: 1257–1265, 2004. [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci 12: 2493–2503, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci 107: 757–769, 1993. [DOI] [PubMed] [Google Scholar]
- Budinger E, Laszcz A, Lison H, Scheich H, Ohl FW. Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: bottom-up and top-down processing of neuronal information via field AI. Brain Res 1220: 2–32, 2008. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience 30: 77–86, 1989. [DOI] [PubMed] [Google Scholar]
- Clement MJ, Dietz N, Gupta P, Kanwal JS. Audiovocal communication and social behavior in mustached bats. In: Behavior and Neurodynamics for Auditory Communication, edited by Kanwal JS, Ehret G. Cambridge, England: Cambridge University Press, 2006, p. 57–84. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry 6: 13–34, 2001. [DOI] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J Comp Neurol 425: 257–274, 2000. [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci 106: 81–105, 1992. [DOI] [PubMed] [Google Scholar]
- Ehret G, Bernecker C. Low-frequency sound communication by mouse pups: wriggling calls release maternal behaviour. Anim Behav 34: 821–830, 1986. [Google Scholar]
- Esser KH, Lud B. Discrimination of sinusoidally frequency-modulated sound signals mimicking species-specific communication calls in the FM-bat Phyllostomus discolor. J Comp Physiol A 180: 513–522, 1997. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: The Amygdala: a Functional Analysis, edited by Aggleton JP. New York: Oxford University Press, 2000, p. 690. [Google Scholar]
- Fenton MB. Communication in the Chiroptera. Bloomington, IN: Indiana University Press, 1985. [Google Scholar]
- Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Neural correlates of auditory stream segregation in primary auditory cortex of the awake monkey. Hear Res 151: 167–187, 2001. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kanwal JS, Butman JA, Suga N. Combination-sensitive neurons in the primary auditory cortex of the mustached bat. J Neurosci 13: 931–940, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Olsen JF, Suga N. Connections among functional areas in the mustached bat auditory cortex. J Comp Neurol 391: 366–396, 1998. [PubMed] [Google Scholar]
- Fricke ST, Vink R, Chiodo C, Cernak I, Ileva L, Faden AI. Consistent and reproducible slice selection in rodent brain using a novel stereotaxic device for MRI. J Neurosci Methods 136: 99–102, 2004. [DOI] [PubMed] [Google Scholar]
- Fung SH, Burstein D, Born RT. In vivo microelectrode track reconstruction using magnetic resonance imaging. J Neurosci Methods 80: 215–224, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115: 1261–1279, 2002. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD. The auditory behaviour of primates: a neuroethological perspective. Curr Opin Neurobiol 11: 712–720, 2001. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD. The neuroethology of primate vocal communication: substrates for the evolution of speech. Trends Cogn Sci 3: 377–384, 1999. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Braun A., Lopes M, Hauser M.D, Carson RE, Herscovitch P, Martin A. Toward an evolutionary perspective on conceptual representation: species-specific calls activate visual and affective processing systems in the macaque. Proc Natl Acad Sci USA 101: 17516–17521, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol 97: 1671–1683, 2007. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav 77: 489–493, 2002. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic “songs' with approach behaviour. Biol Lett 5: 589–592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M. Functional referents and acoustic similarity: field playback experiments with rhesus monkeys. Anim Behav 55: 1647–1658, 1998. [DOI] [PubMed] [Google Scholar]
- Hoy RR. Acoustic communication in crickets: a model system for the study of feature detection. Fed Proc 37: 2316–2323, 1978. [PubMed] [Google Scholar]
- Janik VM. Whistle matching in wild bottlenose dolphins (Tursiops truncatus). Science 289: 1355–1357, 2000. [DOI] [PubMed] [Google Scholar]
- Kamada K, Pekar JJ, Kanwal JS. Anatomical and functional imaging of the auditory cortex in awake mustached bats using magnetic resonance technology. Brain Res Protoc 4: 351–359, 1999. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Fitzpatrick DC, Suga N. Facilitatory and inhibitory frequency tuning of combination-sensitive neurons in the primary auditory cortex of mustached bats. J Neurophysiol 82: 2327–2345, 1999. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Gordon M, Peng JP, Heinz-Esser K. Auditory responses from the frontal cortex in the mustached bat, Pteronotus parnellii. Neuroreport 11: 367–372, 2000. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Matsumura S, Ohlemiller K, Suga N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J Acoust Soc Am 96: 1229–1254, 1994. [DOI] [PubMed] [Google Scholar]
- Katayama T, Jodo E, Suzuki Y, Hoshino KY, Takeuchi S, Kayama Y. Phencyclidine affects firing activity of basolateral amygdala neurons related to social behavior in rats. Neuroscience 159: 335–343, 2009. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388: 377–380, 1997. [DOI] [PubMed] [Google Scholar]
- Kobler JB, Isbey SF, Casseday JH. Auditory pathways to the frontal cortex of the mustache bat, Pteronotus parnellii. Science 236: 824–826, 1987. [DOI] [PubMed] [Google Scholar]
- Konishi M. Pattern generation in birdsong. Neurobiology 4: 827–831, 1994. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Parnavelas JG. Changing concepts of cortical development. Cereb Cortex 13: 541, 2003. [DOI] [PubMed] [Google Scholar]
- Kuraoka K, Nakamura K. Impacts of facial identity and type of emotion on responses of amygdala neurons. Neuroreport 17: 9–12, 2006. [DOI] [PubMed] [Google Scholar]
- Kuraoka K, Nakamura K. Responses of single neurons in monkey amygdala to facial and vocal emotions. J Neurophysiol 97: 1379–1387, 2007. [DOI] [PubMed] [Google Scholar]
- Kusmierek P, Rauschecker JP. Functional specialization of medial auditory belt cortex in the alert rhesus monkey. J Neurophysiol 102: 1606–1622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Pare D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol 77: 341–352, 1997. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Nader K, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of posterior thalamic and cortical lesions on fear conditioning. Neuroscience 125: 305–315, 2004. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10: 1062–1069, 1990a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 1043–1054, 1990b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol 242: 182–213, 1985. [DOI] [PubMed] [Google Scholar]
- Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Mem 3: 229–242, 1996. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav Brain Res 182: 166–172, 2007. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Dopaminergic and non-dopaminergic projections to amygdala from substantia nigra and ventral tegmental area. Brain Res 262: 334–338, 1983. [DOI] [PubMed] [Google Scholar]
- Ma J, Kobayasi K, Zhang S, Metzner W. Vocal communication in adult greater horseshoe bats, Rhinolophus ferrumequinum. J Comp Physiol A 192: 535–550, 2006. [DOI] [PubMed] [Google Scholar]
- Ma J, Naumann RT, Kanwal JS. Fear conditioned discrimination of frequency modulated sweeps within species-specific calls of mustached bats. PLos One 5: e10579, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machens CK, Stemmler MB, Prinz P, Krahe R, Ronacher B, Herz AV. Representation of acoustic communication signals by insect auditory receptor neurons. J Neurosci 21: 3215–3227, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Suga N, Ostwald J. Aural representation in the doppler-shifted-CF processing area of the auditory cortex of the mustache bat. Science 200: 339–342, 1978. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852, 2004. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48: 1025–1037, 2005. [DOI] [PubMed] [Google Scholar]
- Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci 22: 10449–10460, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71: 55–75, 1996. [DOI] [PubMed] [Google Scholar]
- Medvedev AV, Kanwal JS. Local field potentials and spiking activity in the primary auditory cortex in response to social calls. J Neurophysiol 92: 52–65, 2004. [DOI] [PubMed] [Google Scholar]
- Mello LE, Tan AM, Finch DM. Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: an in vivo extra- and intracellular electrophysiological study. Brain Res 587: 24–40, 1992. [DOI] [PubMed] [Google Scholar]
- Morton ES. Grading, dicreteness, redundancy, and motivation-structural rules. In: Acoustic Communication in Birds, edited by Miller K. New York: Academic, 1982, p. 183– 24–212. [Google Scholar]
- Morton ES. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am Nat 111: 855–869, 1977. [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. J Neurosci 52: 621–636, 1993. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem 8: 156–163, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Categorical perception of a natural stimulus continuum: birdsong. Science 244: 976–978, 1989. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci 8: 3570–3583, 1988a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. Topographic distribution of modality-specific amygdalar neurons in alert monkey. J Neurosci 8: 3556–3569, 1988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller K, Kanwal JS, Butman JA, Suga N. Stimulus Design for Auditory Neuroethology: synthesis and manipulation of complex communication sounds. Audit Neurosci 1: 19–37, 1994. [Google Scholar]
- Ohlemiller KK, Kanwal JS, Suga N. Facilitative responses to species-specific calls in cortical FM-FM neurons of the mustached bat. Neuroreport 7: 1749–1755, 1996. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57: 1077–1090, 1993. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439: 865–870, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JP, Kanwal JS. Multipeaked harmonic tuning and call responses of neurons in the “tonotopic” auditory cortex of the mustached bat. In: Proceedings of the 6th International Congress of Neuroethology, edited by Bleckmann H. Bonn, Germany, 2001. [Google Scholar]
- Peterson GE, Barney HL. Control methods used in a study of the vowels. J Acoust Soc Am 24: 175–184, 1952. [Google Scholar]
- Pitkanen A. Connctivity of the rat amygdaloid complex. In: The Amygdala: a Functional Analysis, edited by Aggleton JP. New York: Oxford University Press, 2000, p. 690. [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann NY Acad Sci 911: 369–391, 2000. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523, 1997. [DOI] [PubMed] [Google Scholar]
- Prasada Rao PD, Kanwal JS. Oxytocin and vasopressin immunoreactivity within the forebrain and limbic-related areas in the mustached bat, Pteronotus parnellii. Brain Behav Evol 63: 151–168, 2004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci 4: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci 107: 444–450, 1993. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex 3: 515–532, 1993. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, De Armentia ML, Power J. The amygdaloid complex: anatomy and physiology. Phys Rev 83: 803–834, 2003. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res 764: 262–264, 1997. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. In: Linking Affect to Action: Critical Contributions of the Orbitofrontal Cortex, edited by Schoenbaum G, Gottfried JA, Murray EA, Ramus SJ. Oxford: Blackwell, 2007, p. 336–354. [Google Scholar]
- Sander K, Brechmann A, Scheich H. Audition of laughing and crying leads to right amygdala activation in a low-noise fMRI setting. Brain Res Brain Res Protoc 11: 81–91, 2003. [DOI] [PubMed] [Google Scholar]
- Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res 12: 181–198, 2001. [DOI] [PubMed] [Google Scholar]
- Sander K, Scheich H. Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci 17: 1519–1531, 2005. [DOI] [PubMed] [Google Scholar]
- Schmidt-French B, Gillam E, Fenton MB. Vocalizations emitted during mother-young interactions by captive eastern red bats, Lasiurus borealis (Chiroptera:Vespertilionidae). Acta Chiropterol 8: 477–484, 2006. [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci 19: 1876–1884, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. Eur J Neurosci 22: 1775–1783, 2005. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods 18: 339–350, 1986. [DOI] [PubMed] [Google Scholar]
- Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature 385: 254–257, 1997. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Meaning and emotion in animal vocalizations. Ann NY Acad Sci 1000: 32–55, 2003. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 9: 1028–1035, 2006. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Linguistic and psycholinguistic approaches to primate communication. In: Primate Communication, edited by Snowdon CT, Brown CH, Petersen MR. Cambridge, England: Cambridge University Press, 1982, p. 212–238. [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol 451: 301–323, 2002. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Volkov IO, Fishman YI, Oya H, Arezzo JC, Howard MA., III Intracortical responses in human and monkey primary auditory cortex support a temporal processing mechanism for encoding of the voice onset time phonetic parameter. Cereb Cortex 15: 170–186, 2005. [DOI] [PubMed] [Google Scholar]
- Suga N. Processing of auditory information carried by species-specific complex sounds. In: The Cognitive Neurosciences, edited by Gazzaniga MS. Cambridge: MIT Press, 1994, p. 295– 170–313. [Google Scholar]
- Suga N, Manabe T. Neural basis of amplitude-spectrum representation in auditory-cortex of the mustached bat. J Neurophysiol 47: 225–255, 1982. [DOI] [PubMed] [Google Scholar]
- Suga N, Niwa H, Taniguchi I, Margoliash D. The personalized auditory cortex of the mustached bat: adaptation for echolocation. J Neurol 58: 643–654, 1987. [DOI] [PubMed] [Google Scholar]
- Suga N, O'Neill WE, Manabe T. Harmonic-sensitive neurons in the auditory cortex of the mustached bat. Science 203: 270–274, 1979. [DOI] [PubMed] [Google Scholar]
- Sugase-Miyamoto Y, Richmond BJ. Neuronal signals in the monkey basolateral amygdala during reward schedules. J Neurosci 25: 11071–11083, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci 21: 323–331, 1998. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci 20: 8909–8915, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience 160: 284–294, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci 27: 3937–3945, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Kanwal JS. DSCF neurons within the primary auditory cortex of the mustached bat process frequency modulations present within social calls. J Neurophysiol 100: 3285–3304, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci 25: 4577–4586, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science 306: 2061, 2004. [DOI] [PubMed] [Google Scholar]
- Wilson DR, Hare JF. Animal communication: ground squirrel uses ultrasonic alarms. Nature 430: 523, 2004. [DOI] [PubMed] [Google Scholar]
- Wohr M, Schwarting RKW. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLos One 2: 12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel Y, Melcon ML, Franz MO, Denzinger A, Schnitzler HU. The voice of bats: how greater mouse-eared bats recognize individuals based on their echolocation calls. PLoS Comput Biol 5: e1000400, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.