Abstract
Three monkeys performed a visually guided reach-touch task with and without laterally displacing prisms. The prisms offset the normally aligned gaze/reach and subsequent touch. Naive monkeys showed adaptation, such that on repeated prism trials the gaze-reach angle widened and touches hit nearer the target. On the first subsequent no-prism trial the monkeys exhibited an aftereffect, such that the widened gaze-reach angle persisted and touches missed the target in the direction opposite that of initial prism-induced error. After 20–30 days of training, monkeys showed long-term learning and storage of the prism gaze-reach calibration: they switched between prism and no-prism and touched the target on the first trials without adaptation or aftereffect. Injections of lidocaine into posterolateral cerebellar cortex or muscimol or lidocaine into dentate nucleus temporarily inactivated these structures. Immediately after injections into cortex or dentate, reaches were displaced in the direction of prism-displaced gaze, but no-prism reaches were relatively unimpaired. There was little or no adaptation on the day of injection. On days after injection, there was no adaptation and both prism and no-prism reaches were horizontally, and often vertically, displaced. A single permanent lesion (kainic acid) in the lateral dentate nucleus of one monkey immediately impaired only the learned prism gaze-reach calibration and in subsequent days disrupted both learning and performance. This effect persisted for the 18 days of observation, with little or no adaptation.
Keywords: adaptation, coordination, dentate nucleus, inferior olive, motor learning
studies on the conditioned eyeblink and vestibuloocular reflex in rabbits and rodents have addressed the question of whether these conditioned responses require both cerebellar cortex and deep nuclei or whether the conditioned response is stored in the deep nuclei and timing is stored in the cortex. Questions remain on how the cerebellar cortex and nuclei contribute to performance and learning of more functional behaviors such as the visually guided reach. A number of experiments have shown that novel adaptation of a movement is impaired after cerebellar lesions (Ito et al. 1974; McCormick and Thompson 1984; Thach et al. 1992).1 One such example is adaptation of eye-hand coordination to wedge prisms that displace the visual image (and thus gaze) laterally (Helmholtz 1924–1925). Lesions of cerebellar cortex impair the ability to adapt gaze-arm coordination to prisms in humans (Martin et al. 1996a, Pisella et al. 2005; Weiner et al. 1983; Werner et al. 2010) and monkeys (Baizer and Glickstein 1974; Baizer et al. 1999). After repeated exposure to prisms, normal humans store a second gaze-reach calibration, such that when switching from prism to no-prism and vice versa, subjects hit the target on the first attempt (Martin et al. 1996b).
One question remains as to whether new motor memories, once formed, are localized (Krakauer and Shadmehr 2006), and if so, where—within cerebellar cortex (Gilbert and Thach 1977; Ito et al. 1974; Nagao and Kitazawa 2003; Yeo et al. 1985), within cerebellar nuclei (Anzai et al. 2010; Krupa et al. 1993; McCormick and Thompson 1984; Ohyama et al. 2003), within both cerebellar cortex and nuclei (Mauk and Donegan 1997; Mauk 1997; Nagao and Kitazawa 2008; Perrett et al. 1993; Shutoh et al. 2006), or outside the cerebellum in cerebral cortex (Petersen et al. 1989; Raichle et al. 1994) or brain stem (Porrill and Dean 2007). Lesions of cerebellar inputs or outputs have also been reported to abolish motor memory (Glickstein 1992).
We undertook the present study to address in macaques the question of localization of the motor memory with novel gaze-reach calibrations using laterally displacing wedge prisms. Temporary inactivation of cerebellar cortex was achieved with lidocaine and of the dentate nucleus with muscimol or lidocaine. On the day of inactivation of either the cerebellar cortex or the dentate nucleus, only the prism gaze-reach calibration was impaired. On days following inactivation of either the cerebellar cortex or the dentate nucleus, both the prism and the no-prism gaze-reach calibrations were impaired. Similar effects followed a single kainic acid injection into the white matter just lateral to the dentate nucleus in one monkey.
METHODS
Setup.
All surgical and experimental procedures were in accordance with National Institutes of Health and U.S. Department of Agriculture guidelines and were approved by the Animal Studies Committee at Washington University School of Medicine. Two male and one female rhesus monkey (Macaca mulatta) each performed a visually guided reach-touch task. The monkeys sat in a custom-built primate chair that restrained the head but allowed free movement of eyes and arms. Two capacitance switches on the chair required the monkey to hold its arms at its sides with hands on the switches to initiate a trial. A vertical 15-in. touch-sensitive video screen was placed 17.5 cm from the monkey's eyes such that a 1-mm horizontal distance between two points on the screen represented 0.32° of visual angle. The touch screen presented a randomly located visual target dot and registered the monkey's touch time and location. A 10 mm × 10 mm white square continuously displayed in the upper right corner signified that all reaches from switch to screen were to be made with the right arm (Greger et al. 2004; Norris et al. 2004).
Behavioral task.
Figure 1A shows the task. To initiate a trial, the monkey held both its hands on capacitance switches for a random hold time (500–1,000 ms). A red target dot, 6 mm in diameter, then appeared on the video screen. Following target appearance, the monkey was required to initiate a reach with its right hand within 5,000 ms. Once the right hand left the switch, the monkey had 300 ms in which to touch the screen. When the hand touched the screen, a white dot 6 mm in diameter appeared at the location of touch, while the red target dot remained. The monkey then had 400 ms to return the hand back to the capacitance switch. A random final hold time (between 500 and 1,500 ms) was required before the monkey received a liquid reward for a correct trial and before the target and touch dots disappeared.
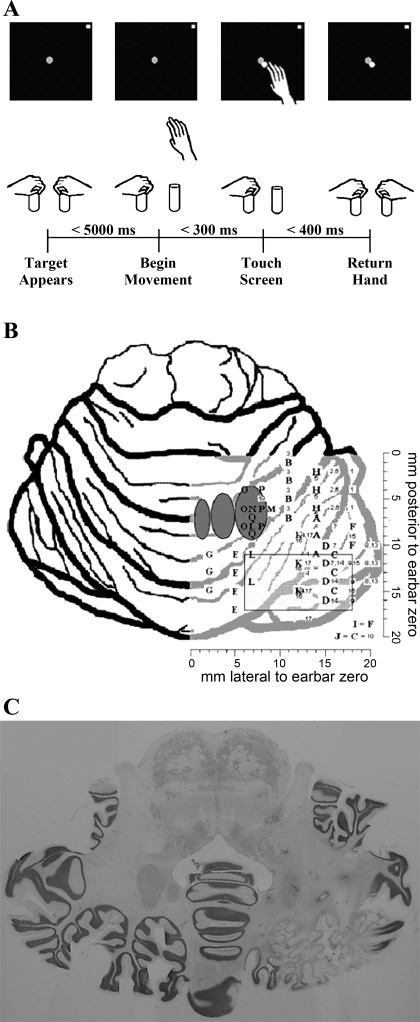
Fig. 1.
A: the sequence of events for a single trial is shown. Trials were completed in blocks with and without prism glasses as described in text. B: graphical depiction of penetration sites in 3 monkeys. Letters correspond to inactivation sites depicted in Table 1 that resulted in a significant change in reach behavior. Numbers correspond to inactivation sites depicted in Table 1 that did not result in significant changes in reach behavior. Dark gray ovals represent fastigial, interposed, and dentate nucleus locations from left to right (medial to lateral). Light gray rectangle represents a grid of saline and muscimol injections (monkey G, injections 11, 12) that did not produce any significant changes in reach behavior. C: postmortem Nissl-stained cerebellar axial cuts from monkey R. These slices demonstrate cellular infiltrates, necrosis of white matter tracts in and just lateral to the dentate nucleus, and marked atrophy of the posterolateral inferior cerebellar cortex.
A trial was considered correct if the target and touch dots overlapped (maximum center-to-center distance of 6 mm). If the target and touch dots did not overlap, the monkey did not receive a reward. During a trial, the hand contralateral to the reach was required to remain on the switch at all times. If this hand was removed from the switch, the trial was immediately aborted and recorded as incomplete. An incomplete trial was also recorded if any of the time constraints were exceeded throughout the reach. After an incomplete trial, the screen was immediately cleared until a new trial began after a 3-s delay.
Trials were performed in alternating blocks of no-prism reaches and prism reaches. In each trial (no-prism or prism), the target appeared at a random location on the screen. For prism reaches, 20 diopter base left (deviating gaze 11.3° to the right) Fresnel prisms (3M Health Care) were placed directly in front of the monkey's eyes (Fig. 1) at a distance of ∼2 cm (monkeys G and R) or mounted 6 cm from the eyes, occluding all visual input beyond it except the screen (monkey C). While distance of the prisms from the eyes may have made some difference in the magnitude of the gaze-reach displacements, the direction of change was the same, and the mounted prisms made training easier.
During training and experimentation, animals were typically required to complete 100 trials before switching between blocks of prism and no-prism reaches. Nevertheless, occasional exceptions were made based on the experimenter's subjective analysis of the animal's motivation. Generally, between 600 and 1,200 reaches were completed each day, allowing the monkey to obtain between 100 and 200 ml of water. There was no maximum limit regarding the amount of trials (and hence water) a monkey could perform in a single session. If the animal was unable to fulfill minimal daily water requirements (30 ml/kg), water was supplemented outside of the experimental setup at random times after completion of the reach session. Failure to successfully touch the target resulted in decreased reward and occasional decreased motivation. As a result, at the experimenter's discretion, water was supplied at variable intervals in all reach conditions when the animal's motivation was questioned (i.e., longer latency between reaches, noncooperation with task parameters, etc.).
Surgical procedures.
Two surgical operations were performed on each monkey 1) to affix an acrylic headholder cap and 2) to place stereotaxically a Lucite recording chamber on the cap (internal proportions of 20 mm2, centered at 10 mm posterior of the interaural line, 10 mm right of midline).
Locating dentate nucleus.
Before inactivation, we recorded single cerebellar units extracellularly to locate the dentate nucleus. Stereotaxic atlases (Snider and Lee 1961; Winters et al. 1969) guided positioning of high-impedance glass-coated platinum/iridium microelectrodes (Frederick Haer) that were mounted on an x,y,z drive aligned with the interaural line and midline. After penetrating dura mater with a beveled guard tube, an electrode passed through the guard tube into the cerebellum. The presence of tonic firing of “simple” spikes periodically interrupted by “complex spikes” (CS) identified Purkinje cells in cerebellar cortex (Thach 1967, 1968). Tonic firing of simple spikes unaccompanied by CS identified deep nuclear cells (Thach 1968). Signals were sent to an AC coupled differential amplifier (gain 10,000; band pass filter 0.1–10 kHz). The analog waveform was sent to an oscilloscope, an audio amplifier/speaker, and an electronic spike discriminator (which digitally sorted simple and complex spikes); the analog and digital signals were stored in computer memory. From recordings of the different cerebellar neurons, we formed a three-dimensional map of hemispheric cerebellar cortex and of anterior, posterior, and lateral borders of the dentate nucleus, as well as its overall depth. This map guided future inactivation attempts aimed at the dentate nucleus.
Inactivation.
In humans, we had observed (Martin et al. 1996a) that large infarcts of the cerebellar hemisphere in the territory of the posterior inferior cerebellar artery impaired adaptation of gaze-arm throw coordination, without disturbing coordination of the arm throw per se. In monkeys, we wanted to inactivate a comparably large volume of cerebellar hemispheral cortex with a minimum number of injections. We chose lidocaine, since it would inactivate not only the nearby neuron somata but also [more sensitively (Sandkuhler et al. 1987)] all axons within a millimeter of the injection and their distal branches to wider regions. These axons would include inferior olive climbing fibers, which branch over several folia in the sagittal dimension; mossy fibers, which branch both sagittally and coronally; and especially the parallel fibers, which run for distances up to 2–3 mm in either direction along the folium after branching from their vertical axon of the granule cell (Mugnaini 1983). Within the deep nuclei, where the cerebellar output projections are focused within a much smaller volume, we wanted to produce a more focal temporary inactivation. We chose the GABA-A agonist muscimol as our primary agent, since the deep cerebellar nuclear cells are richly supplied with GABA-A receptors through which Purkinje axon terminals act to inhibit them. We also made a few injections in the dentate with lidocaine, knowing that the inactivation would be less focal since it would involve not only the cells of the dentate but also all the nearby axons mentioned above. Finally, we chose kainic acid to permanently inactivate inputs to the dentate nucleus.
The lidocaine injected was commercially produced 2% lidocaine hydrochloride solution (Abbott Laboratories), originally buffered at a pH of 6.5–7.0. Before the first injection, the pH of the entire aliquot was adjusted to 7.3 with microliter amounts of NaOH and HCl. The 8.8 mM muscimol solution used for injection was formulated by mixing 1 mg of muscimol (Sigma) in 0.5 ml of physiological saline and adjusting the pH to 7.3 by addition of microliter amounts of NaOH and HCl. Saline was again added so that a final volume of 1 ml was achieved, and the final pH was measured at 7.3 and adjusted if necessary. The 4.8 mM kainic acid solution was formulated in a manner similar to that of the muscimol from 1 mg of kainic acid (Sigma). All solutions remained refrigerated and in the dark between experiments.
An initial reach session (a block of no less than 50 completed no-prism trials followed by a block of no less than 50 completed prism trials) preceded each injection to measure preinjection performance. Then, microinjections were made with a 10-ml GlenCo microsyringe (Spectrum Laboratories) through a 4-in., 26-gauge needle. The syringe was mounted on the same x,y,z drive used previously for single-unit recordings and was sterotaxically aligned within the chamber. After penetrating dura mater with a beveled guard tube, the microsyringe needle was guided through the guard tube to either 1) the skull below cerebellar cortex, then retracted 2 mm, or 2) the deepest location of the dentate nucleus (as determined by prior electrophysiological recordings; see Fig. 1B). At this site, we slowly injected a volume of 2 ml by hand by depressing the plunger, followed by a wait period of no less than 30 s and no greater than 2 min to allow diffusion of the agent. A single injection of 2 ml was generally completed within 30–60 s. We then slowly retracted the needle and repeated the entire process two to four times over a vertical range, injecting at sites 2 mm more superficial to the previous site. Then we made zero to three additional penetrations in the sagittal plane, separated by 2 mm, and the injection scheme repeated, in effect attempting to approximate a surgical “slice” through white matter in the posterior sagittal plane. Diffusion of muscimol has been calculated from behavioral effects in monkey (Thach et al. 1998), cat (Martin et al. 2000), and rat (Arikan et al. 2002) to extend spherically, ∼1 mm from the site of injection. Injection sites were spaced at 2-mm intervals so that the injected material would meet/overlap and form a continuum. Injection locations for all monkeys are represented in Fig. 1B. Table 1 depicts the number of penetrations, the number of injections per penetration, and the volume of solution per injection. After the final injection, the microsyringe was removed, and the monkey was then allowed to continue the reach task.
Table 1.
Injection locations in three monkeys
| Penetration Location |
|||||||
|---|---|---|---|---|---|---|---|
| Injection | Injection Agent | Total No. of Penetrations | Total No. of Injections | Total Vol., μl | Location | Med-Lat, mm | Ant-Post, mm |
| G-A | L | 3 | 9 | 18 | C | 14 | 7, 9, 11 |
| G-B | L | 4 | 12 | 24 | C | 11 | 1, 3, 5, 7 |
| G-C | L | 3 | 9 | 18 | C | 16 | 11, 13, 15 |
| G-D | L | 4 | 12 | 24 | C | 15 | 10, 12, 14, 16 |
| G-1 | L | 3 | 9 | 18 | C | 18 | 2, 4, 6 |
| G-E | L | 4 | 16 | 32 | C | 5 | 11, 13, 15, 17 |
| G-F | L | 2 | 6 | 12 | C | 18 | 8, 10 |
| G-G | L | 3 | 9 | 18 | C | 2 | 11, 13, 15 |
| G-H | L | 3 | 9 | 18 | C | 14 | 2, 4, 6 |
| G-2 | L | 3 | 9 | 18 | C | 16 | 2, 4, 6 |
| G-3 | L | 4 | 12 | 24 | C | 11 | 0, 2, 4, 6 |
| G-4 | L | 4 | 12 | 24 | C | 13 | 9, 11, 13, 15 |
| G-I | L | 2 | 6 | 12 | C | 18 | 8, 10 |
| G-5 | L | 3 | 9 | 18 | C | 14 | 2, 4, 6 |
| G-6 | L | 3 | 9 | 18 | C | 16 | 2, 4, 6 |
| G-7 | L | 3 | 9 | 18 | C | 16 | 8, 10, 12 |
| G-8 | L | 3 | 9 | 18 | C | 20 | 10, 12, 14 |
| G-9 | L | 3 | 9 | 18 | C | 18 | 12, 14, 16 |
| G-10 | S | 3 | 12 | 24 | C | 12 | 9, 12, 15 |
| G-11 | S | 16 | 48 | 96 | C | 6, 10, 14, 18 | 11, 13, 15, 17 |
| G-12 | M | 16 | 48 | 96 | C | 6, 10, 14, 18 | 11, 13, 15, 17 |
| R-J | L | 3 | 9 | 18 | C | 16 | 11, 13, 15 |
| R-13 | L | 3 | 9 | 18 | C | 20 | 10, 12, 14 |
| R-14 | L | 3 | 9 | 18 | C | 16 | 12, 14, 16 |
| R-15 | L | 3 | 4 | 8 | C | 18 | 9, 12, 15 |
| R-16 | L | 4 | 6 | 12 | C | 13 | 9, 12, 15, 18 |
| R-K | L | 3 | 12 | 24 | C | 12 | 9, 12, 15 |
| R-17 | L | 1 | 3 | 6 | D | 8 | 5 |
| R-L | L | 3 | 12 | 24 | D | 7 | 8, 10, 12 |
| C-M | M | 1 | 3 | 6 | D | 9 | 6 |
| C-N | M | 1 | 3 | 6 | D | 7 | 6 |
| C-O | M | 3 | 6 | 12 | D | 6 | 4, 6, 8 |
| C-P | M | 3 | 6 | 12 | D | 8 | 4, 6, 8 |
| R-Q | K | 2 | 4 | 8 | D | 7 | 7, 9 |
Data are total number of injections and total number of penetrations, volume of infusate, and injection locations for all injections in monkeys G, R, and C. Injection agents: L, lidocaine; S, saline; M, muscimol; K, kainic acid. Penetration locations (C, cortex; D, dentate) represent medial-lateral (Med-Lat) and anterior-posterior (Ant-Post) coordinates relative to earbar (0,0) as depicted in Fig. 1B. Inactivations that produced significant reach deficits are labeled with corresponding letters, while inactivations that did not produce any significant reach deficits are depicted numerically.
Histology.
The monkeys were killed with an overdose of intravenous Nembutal followed by an intracardiac perfusion of normal saline, heparin, and 10% formalin in phosphate buffer. The chamber coordinates were marked by stereotaxically aligning a 28-gauge probe coated with dye on the center of the chamber and guiding it through the dorsal-ventral extent of the cerebellum in situ. The cerebellum was removed, sectioned in 100-μm horizontal slices (monkey G, celloidin embedded) or 50-μm horizontal slices (frozen section, monkeys R and C) and Nissl stained.
Analysis.
Behavioral data were recorded and sent to the task control PC, where analysis was conducted off-line. Mean values were calculated for success rate, reach time, and return time and were tested across various behavioral conditions for statistically significant differences between correct and incorrect trials and between reaches conducted in the no-prism condition and prism reaches (Student's t-test, P < 0.05).
Definition of “motor learning.”
In preinjection trials, we defined operationally that motor learning had occurred if (before inactivation) there was no significant difference between the mean horizontal deviation of the first five reaches in a prism block and the first five reaches in a no-prism block for five consecutive days.
Definition of “motor adaptation.”
In determining within a block of trials whether adaptation occurred, we tried to fit each block of prism trials to the regression equation y = a − b × e−t/c, where a is the final value that the exponential decay function approaches, b is the magnitude of the adaptation required from the first reach to the value a, c (the exponential decay constant) represents the rate at which adaptation takes place [adaptation coefficient (AC)], and t is the trial number (Martin et al. 1996a). A decay constant (c) of <1 in the model equation demonstrated that there is no adaptation because the fit approximated a straight line. We also tested for adaptation by looking for a significant difference between the means of lateral displacement of the first five touches and the last five touches within a block of trials [no-prism or prism—Mann-Whitney U-test (MWU), special table for small n; Darlington and Nathan 1975]. If no significant difference existed between these means, we concluded that adaptation did not take place.
RESULTS
Prism learning.
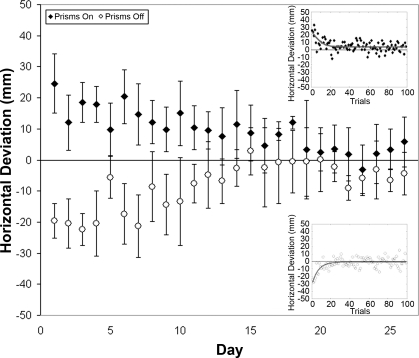
Before inactivations, monkeys G, R, and C trained to perform the reach-touch task with and without prisms. Performing with prisms for the first time, the monkeys' mean touch displacements for the first five reaches were 22.2 mm (monkey R) and 24.6 mm (monkey C) to the right of the target. Figure 2 (top right inset) shows this first day of adaptation for monkey C. The exponential decay curve y = a − b × e−t/c was fit to the prism data, and the AC (= c) was measured to be 7.7 (monkey C) and 8.4 (monkey R)—indicating that it took ∼8 trials to reach a point (1 − e−1) ∼63.2% of the way through adaptation (Martin et al. 1996a). Removal of the prisms on this first day of performance resulted in a “negative aftereffect” (Martin et al. 1996a). This initial aftereffect was measured as the mean of the first five touches upon removal of prisms, and was 25.4 mm (monkey R) and 19.6 mm (monkey C) to the left of the target. The exponential decay curve y = a − b × e−t/c was fit to a data set created by applying the opposite sign to the original data set. The opposite sign was then applied to all calculated curve data, and these data were overlaid with the original data set to create a plot of the aftereffect. Figure 2 (bottom right inset) shows the fitted exponential function for this initial aftereffect in monkey C.
Fig. 2.
Mean horizontal deviation for the first 5 reaches with prisms donned and for the first 5 reaches after removal of prism glasses for each day of the initial 26-day training period in monkey C. Positive values on the y-axis represent deviations to the right of target, and negative values represent deviations to the left of target. Top right inset demonstrates horizontal deviation for each trial on day 1 of prism exposure (prism on). Bottom right inset demonstrates horizontal deviation for trials immediately after removal of prisms on day 1 (no prism).
During weeks of practice, the monkeys' first touches with prisms and the first touches without prisms came progressively closer to the target, eventually hitting it. Ultimately, when the monkey switched from prism to no-prism and vice versa, there was no significant difference between the prism and the no-prism mean touch locations. We thus considered that the monkeys had learned the second (prism) gaze-reach calibration and had retained the practiced (no-prism) calibration, storing the two memories independently. We chose five consecutive days in which there was no significant difference between the first five prism touch locations and the following first five no-prism touch locations (MWU P > 0.05) to define that learning was complete. Learning of the prism gaze-reach calibration was complete in 27 days for monkey R and in 23 days for monkey C. Data from the initial training phase for monkey G were lost because of errors in computer transfer and storage, but data from 5 days before inactivation and throughout all phases of experimentation remained intact for analysis.
Preinactivation performance.
For the day before the first inactivation in each monkey, data for the preinactivation performance are presented in Table 2. Reach and return times are not shown for the prism condition because of space limitations. Nevertheless, these values were not significantly different from those shown for the no-prism conditions in all three monkeys (P > 0.05, Student's t-test). Additionally, reach and return times did not significantly differ between correct trials and incorrect trials (P > 0.05, Student's t-test).
Table 2.
Preinactivation behavior in three monkeys
| Horizontal Deviation |
Vertical Deviation |
|||||||
|---|---|---|---|---|---|---|---|---|
| No-prism x, mm | Prism x, mm | No-prism y, mm | Prism y, mm | Reach Time No-Prism, ms | Return Time No-Prism, ms | Correct No-Prism, % | Correct Prism, % | |
| G | −0.53 ± 5.76 | 1.09 ± 7.44 | 1.08 ± 5.59 | 1.04 ± 6.91 | 189 ± 21 | 276 ± 26 | 65 | 56 |
| R | 0.32 ± 4.90 | 0.09 ± 7.10 | 0.35 ± 5.06 | −0.21 ± 4.30 | 148 ± 17 | 281 ± 37 | 61 | 55 |
| C | −1.43 ± 7.01 | 1.05 ± 5.43 | 1.72 ± 6.40 | 1.20 ± 6.13 | 139 ± 18 | 272 ± 40 | 54 | 52 |
Behavioral data collected on the day before the first inactivation in monkeys G, R, and C. Values are means ± SD and % of correct trials.
Cerebellar cortex (lidocaine): immediate impairment of learned prism gaze-reach calibration and sparing of the no-prism calibration.
On the day of injection, before each injection series in each monkey (day 0), a block each of prism and no-prism trials were completed to establish that behavior had recovered from all previous injections. Our criterion was that there be no significant differences between the horizontal component of the first five prism touch locations and the first five no-prism touch locations (MWU P > 0.05).
Lidocaine was then injected into the cerebellar cortex. This was done for 18 injection series in monkey G and 6 in monkey R (Table 1). Eleven of these 24 injection series produced significant reach-touch errors compared with preinjection behavior (Table 3). We concluded that these 11 injection series succeeded in inactivating cerebellar tissue. Figure 3 shows the effects of lidocaine injection D in the posterior lateral cerebellar cortex of monkey G. Before injection, prism and no-prism touches were approximately on target and did not differ significantly from each other. After inactivation, prism touches were consistently and significantly displaced to the right of target (toward gaze), while no-prism reaches remained on or near target. This figure thus demonstrates an initial loss of the learned prism gaze-reach calibration with sparing of the no-prism calibration. This distinction persisted across all blocks of trials for day 0. During the erroneous prism reaches, the monkey showed no tendency to adapt closer to target as demonstrated by an inability to fit the exponential decay curve y = a − b × e−t/c to the data. This failure to adapt was unlike the monkey's first exposure to prisms, at which time it adapted quickly. In fact, in the example shown, there was a slight tendency for both prism and no-prism reaches to gradually deviate further right of target during successive reaches.
Table 3.
Immediate inactivation effects for injections in three monkeys
| Horizontal Deviation |
Vertical Deviation |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Injection | Agent | Location | No-prism x, mm | Prism x, mm | dif, mm | No-prism y, mm | Prism y, mm | dif, mm | Duration, days |
| G-A | L | C | −1.2 ± 6.3 | 17.6 ± 8.9 | 18.8 | 3.4 ± 7.1 | 12.8 ± 8.4 | 9.4‡ | 2 |
| G-B | L | C | 0.2 ± 7.1 | 8.9 ± 7.5 | 8.7 | 2.4 ± 6.9 | 3.0 ± 7.3 | 0.6 | 1 |
| G-C | L | C | −1.7 ± 5.3 | 11.1 ± 9.1 | 12.8 | 2.3 ± 5.6 | 2.9 ± 6.9 | 0.6 | 1 |
| G-D | L | C | 2.4 ± 7.2 | 14.4 ± 3.4 | 12.0 | 1.6 ± 6.8 | 1.6 ± 6.7 | 0.0 | 0 |
| G-E | L | C | −3.1 ± 7.4 | 12.1 ± 6.4 | 15.2 | 3.5 ± 9.5 | 9.6 ± 11.4 | 6.1‡ | 0 |
| G-F | L | C | 3.8 ± 5.9 | 15.4 ± 7.3 | 11.6 | 2.3 ± 5.6 | −2.1 ± 6.6 | −4.4 | 1 |
| G-G | L | C | −0.4 ± 5.3 | 17.6 ± 6.5 | 18.0 | −0.4 ± 8.3 | −0.3 ± 5.8 | 0.1 | 1 |
| G-H | L | C | −1.1 ± 7.2 | −0.7 ± 8.1 | 0.4* | 0.5 ± 6.0 | 0.8 ± 6.5 | 0.3 | 0 |
| G-I | L | C | 0.3 ± 6.1 | 5.9 ± 7.4 | 5.6 | −1.4 ± 6.5 | −7.4 ± 6.4 | −6.0‡ | 2 |
| R-J | L | C | 1.7 ± 5.4 | 10.8 ± 8.4 | 9.1 | −2.1 ± 5.9 | −3.7 ± 6.7 | −1.6 | 1 |
| R-K | L | C | −0.3 ± 6.8 | 7.3 ± 6.3 | 7.6 | 0.6 ± 7.1 | 1.6 ± 6.3 | 1.0 | 1 |
| R-L | L | D | 0.5 ± 9.9 | 8.2 ± 11.1 | 7.7 | −1.1 ± 8.4 | −2.3 ± 9.1 | −1.2 | 1 |
| C-M | M | D | −0.9 ± 10.9 | 2.4 ± 10.6 | 3.3* | 4.6 ± 9.2 | 4.5 ± 10.6 | −0.1 | 0 |
| C-N | M | D | −2.6 ± 10.3 | 9.6 ± 10.2 | 12.2 | 4.4 ± 7.1 | 3.8 ± 8.4 | −0.6 | 1 |
| C-O | M | D | −6.8 ± 11.1 | 6.8 ± 9.9 | 13.6 | −14.8 ± 9.3 | −14.5 ± 9.2 | 0.3 | 1 |
| C-P | M | D | −2.9 ± 9.1 | 21.2 ± 11.6 | 24.1 | 7.6 ± 7.6 | 7.3 ± 8.4 | −0.3 | 2 |
| R-Q | K | D | 7.5 ± 15.3 | 22.7 ± 17.6 | 14.5 | −21.8 ± 15.4 | −22.3 ± 16.7 | 0.5 | 18† |
Mean ± SD data are shown for blocks of trials immediately following injections that produced significant (P < 0.05) changes in reach-touch location compared with reaches made before injection. Injections were made in monkeys G, R, and C at penetration sites A–Q in Fig. 1B. Injections of lidocaine (L), muscimol (M), or kainate (K) were made in cerebellar cortex (C) or dentate nucleus (D). Horizontal deviation is represented for the no-prism block, the prism block, and the difference (dif) between no-prism x and prism x. Vertical deviation is represented for the no-prism block, the prism block, and the difference (dif) between no-prism y and prism y. The duration (injection day =1 day) for which there was a significant displacement of the prism reach/touch horizontal displacement relative to the no-prism reach/touch horizontal displacement (P < 0.05) is also shown.
Horizontal displacement that did not differ significantly across prism and no-prism conditions after injection.
Kainate produced a permanent lesion and displaced reach/touch for the entire period of observation (18 days).
Significant vertical deviation.
Fig. 3.
Horizontal deviation for all trials on a day in which lidocaine was used to inactivate cerebellar cortex in monkey G (injection D). Positive values on the y-axis represent deviations to the right of target, and negative values represent deviations to the left of target. All reaches before injection (labeled vertical bar) land centered near the target, while reaches after injection are markedly to the right of target when prisms are donned. Means ± SD are shown below each block of prism and no-prism reaches.
In this single example, the horizontal touch locations within and across consecutive prism or consecutive no-prism blocks subjectively trended relatively further to the right of target. However, there were no significant differences between mean horizontal touch locations across blocks (Student's t > 0.05) or mean horizontal touch location of the first five reaches compared with the mean of the last five reaches within a single prism or no-prism block after injection (MWU, P > 0.05). A similar subjective trend was observed after two other injections (injection F in monkey G and injection K in monkey R). In these examples, there were significant differences between the mean horizontal touch locations of the first and last prism and no-prism blocks after injection (Student's t-test, P < 0.05) but not on consecutive prism or no-prism blocks after injection.
Table 3 summarizes data immediately following injections on day 0 for errors made in the first blocks of prism and no-prism reaches. Of the 11 successful inactivations of cerebellar cortex that produced significant errors, 10 caused significant loss of the prism gaze-reach calibration without loss of no-prism calibration (P < 0.05, Student's t-test). Furthermore, within blocks of prism and no-prism trials, data could not be fitted with the exponential decay function. This signified a loss of the short-term (within trial block) adaptation in addition to the loss of the long-term learning. Duration of the immediate inactivation effect was defined as the number of days (day 0 = injection day) for which a significant difference existed between the mean horizontal locations of prism and no-prism touches. The duration of the immediate inactivation effect was never greater than 2 days after injection, and is represented in the far right column of Table 3. Occasionally, monkeys would have decreased motivation to continue the task due to decreased ability to correctly hit the target and thus receive reward. As a result, blocks of prism reaches were occasionally reduced from the typically required 100 trials. Mean data presented in Table 3 were calculated for blocks of prism and no-prism data that contained a minimum of 50 completed trials.
Immediately after all lidocaine injections into cerebellar cortex, vertical deviation did not significantly differ in the no-prism reaches compared with baseline reaching. However, during prism reaches, injections resulted in a significant vertical deviation compared with baseline and no-prism reaches. Two of these injections resulted in a vertical deviation above the target, and one resulted in a vertical deviation below target.
The locations of injection series that produced no significant effects on touch location are represented as numbers in Fig. 1B. Some of these injections were located in the same (x,y) areas of cerebellum that did produce significant inactivation results (labeled with letters). All injections are presented chronologically in Table 1.
Saline and muscimol injections controlling for the inactivation effects of cortical lidocaine.
We wanted to test that the inactivation from lidocaine was not simply due to tissue damage of the injection process. Saline was therefore injected into the same site (monkey G, injection 10) where a prior injection of lidocaine (monkey G, injection C) had produced a large inactivation effect (see Fig. 1B). Similarly, saline was injected in monkey R (injection 16) and monkey C (injection 19) preceding injection with lidocaine (injection K) and muscimol (injection O). There was no significant immediate or delayed (see below) change in prism or no-prism calibration after the saline injections, but there were significant changes in reach behavior after later lidocaine in cortex (injection K) and muscimol in dentate (injection O). Additionally, we made multiple saline injections (monkey G, injection 11) in a grid of 16 vertical penetrations that spanned the area of posterolateral cerebellar cortex in which more focal injections of lidocaine had altered the prism/no-prism calibrations (shown as a rectangle in Fig. 1B). Again, there was no significant change in prism or no-prism calibration after the large-volume injection.
Finally, we wanted to control for the effects of lidocaine, which we hypothesized would be directed both at cell bodies and at all axons of passage, by injecting muscimol, which we hypothesized would be directed at cell bodies only. We therefore made multiple muscimol injections (monkey G, injection 12) in the same grid of 16 vertical penetrations used days before for saline injection (same rectangle in Fig. 1B). Surprisingly, we found no significant change in prism or no-prism calibration or in gait following this comprehensive muscimol injection into cerebellar cortex.
Gaze-reach calibration further depends on the location of target appearance.
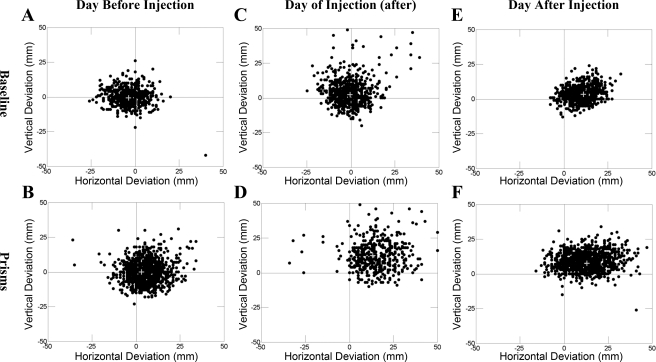
For injections A, E, and I in monkey G, we discovered a bias in errors that were proportionate to the absolute location of the target appearance on the screen. Figure 4 shows the target appearance location for correct and incorrect trials for cortical lidocaine injection E in monkey G. Before inactivation, the distribution of correct and incorrect trials was randomly distributed throughout the entire screen (Fig. 4, A–C). However, on day 0, immediately after inactivation, the monkey made correct targeted reaches preferentially to targets that appeared in the upper right portion of the screen but missed on reaches to the lower left (Fig. 4D). This was especially so for prism reaches. On day 1, subsequent to the inactivation, this spatial sparing to the upper right was maintained for both no-prism and prism reaches (Fig. 4, E and F). For targets appearing in the lower screen, the monkey consistently reached incorrectly above and to the right of the target. Against the possibility of a visual perceptive deficit in the lower left quadrant (possibly resulting from penetration through right parietal cerebral cortex), the monkey always reached to targets in the lower left quadrant, but missed.
Fig. 4.
(x,y) touch points (relative to target appearance) shown separately for no-prism (top) and prism (bottom) trials. Data are shown for all completed trials. A and B: trials completed for the day before lidocaine inactivation of cerebellar cortex in monkey G (injection E). C and D: data for only trials immediately following injection of cerebellar cortex with lidocaine. E and F: relative touch data for the day following inactivation of cerebellar cortex.
Cerebellar cortex (lidocaine): delayed impairment of both no-prism and prism gaze reach calibrations.
For 10 of 11 injections, inactivation that resulted in the loss of the learned prism reach calibration on the day of injection (day 0) generally persisted with addition of a deviation of the no-prism calibration. Immediately after right-sided injection, the no-prism reaches remained near target while prism reaches deviated to the right of target (in the direction of gaze). On day 1 after injection there was no significant difference between the mean location of touches in prism and no-prism blocks of trials, which in turn were now both significantly displaced from the preinactivation touch locations. Two lidocaine injections in cortex (B and H) resulted in a delayed horizontal deviation to the left of target, whereas the remaining injections (9) resulted to the right of target in both prism and no-prism reaches. Of note is the fact that injections B and H were more anterior than the remaining injections into posterior-lateral cerebellar cortex (Fig. 1B). The delayed effect also resulted in 9 of 11 injections deviating vertically from the target in both the prism and no-prism conditions (P > 0.05, Student's t-test). Of these, three deviated below the target and five deviated above target for both prism and no-prism reaches. A single injection (C) resulted in a significant delayed vertical deviation that differed in the prism (above) and no-prism (below) conditions.
Cerebellar dentate nucleus: temporary inactivation with muscimol or lidocaine.
A total of four muscimol (monkey C) and one lidocaine (monkey R) injections were aimed at the dentate nucleus. On the day of injection (day 0), three of four muscimol injections and the lidocaine injection resulted in immediate loss of the learned stored prism calibration without affecting the no-prism reaches (P < 0.05, Student's t-test). No adaptation was detected during the impaired prism trial blocks. These results were thus similar to the immediate effect observed after inactivation of cerebellar cortex. On subsequent days, four of four muscimol injections and the lidocaine injection resulted in a delayed effect of horizontal and vertical touch displacement from the target for both prism and no-prism reaches, again similar to the cortical inactivations (however, injection N, oddly enough, affected the no-prism reach more than the prism reach). While these results appear similar to the delayed impairment observed following inactivation of cerebellar cortex, there were differences. Specifically, three of four muscimol (including N) and the lidocaine deep nuclear injections resulted in delayed reaches being left of target and to the left of the direction of gaze. This was true for only the delayed effect. These results thus differed from inactivation of cerebellar cortex, where most of the lidocaine injections produced both immediate and delayed displacement of touches to the right of target. Data for injections into dentate are shown in Table 3.
Dentate nucleus: permanent inactivation using kainate.
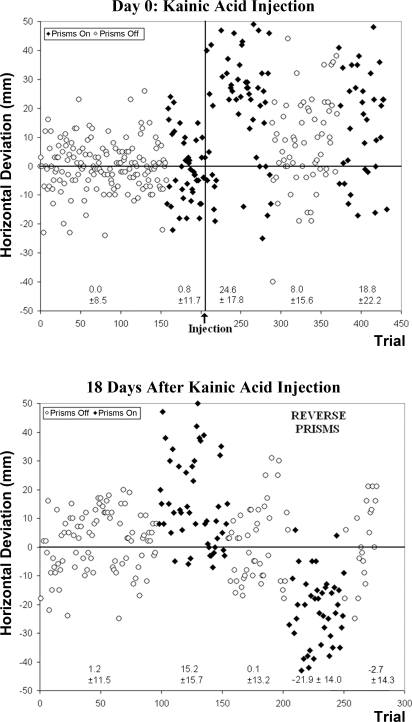
Finally, to clarify the ambiguities about temporary dentate inactivation, we injected kainic acid in the lateral dentate nucleus of monkey R (injection Q). This initially (day 0) produced marked misreach to the right of target with prisms on. There was also an immediate rightward bias present in the no-prism condition, but not to the degree of that observed in the prism condition. Additionally, marked vertical error was observed below the target in both prism and no-prism reaches immediately following kainate injection. On subsequent days the permanent inactivation produced a horizontal deviation only for prism reaches and a vertical deviation for both prism and no-prism reaches. These deficits persisted for 19 days, when the monkey was killed (Fig. 5). Furthermore, Fig. 5 shows little or no adaptation (the exponential decay function y = a − b × e−t/c could not be fit to postinjection prism block reach-touches).
Fig. 5.
Horizontal deviation for all reaches on the day of permanent inactivation with kainic acid (top) and for reaches 18 days following the same injection (bottom) in monkey R, injection Q. Positive values on the y-axis represent deviations to the right of target, and negative values represent deviations to the left of target. Immediately after injection of kainic acid, reaches with prisms donned missed the target markedly to the right of target. Although training with and without prisms occurred between the day of injection and the 18th day following injection (not shown), reaches remained to the right of target with prisms donned 18 days following permanent inactivation. Adaptation was absent or severely impaired when the monkey was exposed to learned prisms and reverse prisms after inactivation. Means ± SD are shown below each block of prism and no-prism reaches.
After permanent inactivation, the prism base was occasionally reversed during reach sessions. When donning reverse prisms, the monkey's gaze was deviated in a direction opposite to that of the learned prisms. This maneuver was conducted in an effort to test the monkey's ability to adapt to a novel visual perturbation, one independent of the learned prism shift direction. Prisms were reversed throughout a single prism block in each of four sessions over the course of the 19-day postinjection period (on postinjection days 4, 10, 14, and 18). Use of reverse prisms was minimized in an effort to reduce learning. After the permanent inactivation, the monkey was unable to adapt to the reverse prisms over the course of the 19 days after injection (Fig. 5), as determined by the inability to fit the exponential curve to the data set and absence of an aftereffect. Because we lacked a baseline measure for reverse prisms, we are unable to determine whether a direction-specific effect occurred. However, the magnitude of error for the initial five reverse-prism trials was similar to the error that the same monkey displayed on its initial five prism trials (23.4 mm compared with 22.2 mm).
Impaired coordination (ataxia) after inactivation.
We defined incoordination (ataxia) as trial-to-trial horizontal or vertical irregularities in reach/touch locations. Ataxia resulted immediately after a total of 5 of 11 temporary inactivations into cerebellar cortex, as measured by an increased standard deviation in the (x,y) distribution centered around target of the trial-by-trial touch error compared with preinjection data. Similarly, ataxia immediately followed a total of five of five temporary inactivations in the dentate nucleus, producing significant trial-to-trial irregularities of touch location in both vertical and horizontal dimensions on both no-prism and prism conditions compared with preinjection data. Measures in the horizontal dimension were significantly greater for the dentate than for the cortex (Table 3; t-test, P < 0.05). Ataxia resulting from injection of lidocaine or muscimol into dentate nucleus resolved the day after injection, as demonstrated by reduction in the standard deviation of mean horizontal and vertical displacement. Consistent with the permanent effect of kainic acid, ataxia in the single permanent lesion in the lateral dentate nucleus persisted. Ataxia was found again to be independent of impairments of the gaze-reach calibration learning and adaptation. (Martin et al. 1996a).
Histological analysis.
Postmortem histological analysis revealed that about half the injection tracts were marked by cellular infiltrates. No other necrosis or hemorrhage was observed. In monkey R, the kainic acid injection (Q) resulted in necrosis of white matter tracts in and just lateral to the dentate nucleus. Additionally, marked atrophy of the posterolateral inferior cerebellar cortex was noted (Fig. 1C) in this monkey, consistent with previous observations of delayed indirect effects of kainic acid resulting in severe loss of postsynaptic neuronal elements (Moriizumi et al. 1991) via axonal connections (Schwob et al. 1980).
Postmortem comparison of histology (see Madigan and Carpenter 1971) to targeted stereotaxic coordinates (Snider and Lee 1961) allowed us to evaluate targeted areas for each injection. While unable to fully account for the extent of diffusion for injected material or any possible cannula deviation once passed through cerebral tentorium, cortical injections were aimed at crus I and/or crus II (injections A, C, D, F, I–K), paramedian lobule (injection E), dorsal paraflocculus (injection K), copula of the pyramis (injection G), and anterior quadrangular lobule (injections B and H, likely affecting lobules V/VI and VI/VII, respectively). All injections targeted at dentate appeared to strike the target except injection M, which extended into the white matter just lateral to dentate.
DISCUSSION
Immediate impairment of learned prism gaze-reach calibration.
Others have proposed that inactivation of cerebellar cortex (Nagao and Kitazawa 2003; Yeo et al. 1985) or of the deep nuclei (McCormick and Thompson 1984; Ohyama et al. 2003; Shutoh et al. 2006) results in loss of stored motor memories of conditioned responses. Thus inactivation of these structures would temporarily disrupt both motor control and any modification these circuits are capable of for the several hours of pharmacological action of the drugs. If only the prism gaze-reach calibration were specifically abolished, one might have expected to observe 1) prism reaches shifted toward gaze, 2) no-prism reaches on target, and 3) adaptation blocked. The observations on the day of injection, immediately following injection, are consistent with these expectations, thus suggesting a specific loss of the learned prism calibration while supporting previous knowledge that lesions in the cerebellum produce deficits in prism adaptation in humans (Martin et al. 1996a; Pisella et al. 2005; Weiner et al. 1983) and monkeys (Baizer and Glickstein 1974; Baizer et al. 1999).
Localization of learned gaze-reach coordination in the cerebellum.
In one of five monkeys trained in a similar prism-reach task, Baizer et al. (1999) reported that a cerebellar lesion that included the dorsal paraflocculus and uvula abolished completely the normal prism adaptation for the arm ipsilateral to the lesion. The other four animals retained the ability to prism-adapt normally and showed the expected postadaptation shift. In the one case in which the lesion abolished prism adaptation, the damage also included crus I and II, paramedian lobule, and the dorsal paraflocculus of the cerebellar hemispheres (similar to the present data) as well as lobule IX of the vermis. Thus, in this case, the lesion included virtually all the cerebellar cortex that receives mossy fiber visual information relayed via the pontine nuclei from the cerebral cortex. The other four animals had damage to lobule V, the classical anterior lobe arm area, and/or vermian lobules VI/VII, the oculomotor region. When tested postoperatively, some of these animals showed a degree of ataxia equivalent to that of the case in which prism adaptation was affected, but prism adaptation and the postadaptation shift remained normal. This was not the case in our present inactivation of posterior lateral cerebellar cortex, thus supporting that damage specific to the posterior-lateral cerebellum affects both learned prism calibration and adaptation.
In a study of humans (Martin et al. 1996a), damage impairing prism adaptation lay within the territory of the posterior inferior cerebellar artery (which includes crus I and II and the lateral inferior dentate). Cerebellar lesions within the superior cerebellar artery territory (which includes anteromedial cerebellar cortex, interposed and superior dentate) caused limb ataxia without impairment of prism adaptation of throwing. These observations appear to agree with the one monkey of Baizer et al. (1999) and our present temporary inactivation of primarily crus I and II of cerebellar cortex. The limited performance errors in our study differ from prior lesion studies in humans demonstrating impaired adaptation and performance following damage to the SCA territory (Pisella et al. 2005; Werner et al. 2010). Because of our technique of injecting columns of inactivating agent in an extensive inferior-superior plane and an inability to differentiate conscious strategy versus true adaptation in monkeys, we are unable to further delineate whether there may be a specific role for the superior cerebellum in true adaptation (visuomotor recalibration—as observed by deficits in aftereffect) versus the posterior inferior cerebellum in strategic change (Werner et al. 2010).
Recent brain imaging studies in healthy human subjects report cerebellar involvement in visuomotor adaptation in the superior cerebellum only (Della-Maggiore and McIntosh 2005; Seidler and Noll 2008; Seidler et al. 2006), in both the superior and posterior inferior cerebellum (Nezafat et al. 2001), or primarily in the anterior arm areas (lobules V, VI, VIII and dentate; Diedrichsen et al. 2005). Again, data in the present study are not sensitive enough to localize superior versus inferior regions. However, involvement of primarily crus I and II in the present study indicates possible limitations in spatial resolution using such imaging techniques (i.e., Diedrichsen et al. 2005). Moreover, lesion studies are important in establishing a casual link for functional MRI (fMRI) findings, since fMRI cannot always demonstrate that an activated area is essential for a given task (Rorden and Karnath 2004). One must also consider species and task differences between such studies. Also, specific to our task, we were monitoring changes to an overly trained learned behavior, which may represent possible dynamic shifts in localization of learned behavior versus adaptation (Nezafat et al. 2001). Concurrent inactivation and imaging studies would be useful in further evaluating such discrepancies in more specific cortical localization.
A permanent lesion with kainic acid was confirmed on histology to involve the lateral aspect of the dentate nucleus. This injection resulted in not only direct damage to cellular components but also extensive atrophy in posterolateral areas affecting crus I and II as well as lateral aspects of the dorsal paraflocculus. This atrophy most likely results from damage/destruction of outputs (Purkinje cells) and inputs (mossy fibers and climbing fibers) to cortex via axonal connections (Schwob et al. 1980). This supports involvement of the ventral dentate nucleus, which receives its primary inputs from said areas. We are unable to differentiate whether temporary inactivations (which were aimed as vertical columns flanking the permanent lesion) primarily involved ventral portions of the dentate receiving inputs from the posterolateral cerebellum or the more dorsal “motor” inputs.
No adaptation during the postinactivation period.
If, in the present study, only the learned prism gaze-reach calibration were specifically abolished, one might have expected that on the day or two following inactivation adaptation would occur so as to “relearn” the prism gaze-reach calibration, and prism memory and performance would be restored. Observations on the many days following injection are not consistent with this explanation. For most injections that resulted in selective impairment of the learned prism gaze-reach calibration, the horizontal displacement for the first five and last five reaches (prism and no-prism) in the days following injection was not statistically different in the direction of target (MWU, P > 0.05). This is consistent with there being no adaptation on those days. The same was true for the kainic acid injection in monkey R that was observed over 19 days. However, for four injections [G, H, I in monkey G (cortical lidocaine) and L in monkey R (dentate lidocaine)], the horizontal displacement was significantly closer to target between the first five and last five prism reaches (MWU, P < 0.05) in the day following injection. This would seem consistent with there being adaptation on those days. Alternatively, the appearance of within-session adaptation on those days following injection may reflect the monkey's conscious strategy to correct and compensate for errors (Redding et al. 2005). From our data, we therefore cannot be confident as to the exact nature of these adjustments in behavior during delayed inactivation effects. Further experiments are required to separate these two mechanisms of postinjection behavior.
Directional bias of impairment.
After select injections, inactivation of right cerebellar cortex resulted in impairment of reaches (with the ipsilateral limb) to the side contralateral the lesion (Fig. 4). This occurred with concurrent best-adapted performance for reaches to the right of screen. This observation may imply something other than pure ipsilateral cerebellar control of arm movement and/or eye-hand motor space. It remains possible that plastic changes occurring in the contralateral cerebellum account for the delayed rightward error in prism and no-prism reaches. Soteropoulos and Baker (2008) demonstrate the role of the interposed nucleus in accessing limb muscles bilaterally and representing bilateral movement. However, the present study as well as the work by Soteropoulos and Baker do not control for eye movement, necessitating future experiments to test this possibility.
Significance of delayed impairment?
The present study was designed to test the effects of short-term, reversible inactivation of cerebellar structures. Thus the hypothesized outcome was that preinjection behavior returned the day following injection (whether spontaneously or via adaptation), after the effects of the pharmacological agents had worn off. This was not the case, as delayed effects occurred in both the prism gaze-reach and no-prism calibrations, both in the horizontal and vertical directions. These effects lingered anywhere from 1 to 7 days following injection and were not associated with increased variance (as was observed following the single kainate injection).
Possible explanations for this include intrinsic structural damage such as neuronal injury produced by insertion of the needle, microhemorrhage not detected on histology, osmotic damage secondary to injection media (despite attempts at physiological preparation), or vasogenic edema. Many of the control injections (including the large-volume saline injection) followed injections of active substance, and thus may have entered areas of previously damaged tissue. Only two control injections [injection 16 (saline); injection 19 (muscimol)] preceded injections of active substance (lidocaine) that later resulted in altered reach behavior. While structural damage may account for the observed delayed effects, additional explanations might entail more complex long-term functional synaptic changes resulting from temporary inactivation. This seems plausible, as lidocaine injections into cerebellar cortex would inactivate all cellular components, including the input (teaching) signals via the climbing fiber, parallel fiber inputs to the Purkinje cell, and Purkinje cell output axons, thus ultimately disinhibiting cerebellar nuclear cells. Independent of etiology (permanent structural vs. temporary functional changes) of delayed effects, such observations have implications for underlying cerebellar function.
Kenyon et al. (1998) have proposed in a computational model that there is a cerebellar-olivary equilibrium resulting in highly regulated spontaneous climbing fiber activity. This is presumed to prevent unwanted synaptic changes and therefore maintain stability of stored memory. Thus it is possible that, even after lidocaine wears off, the circuit synaptic strengths readjusted by this short period of decreased climbing fiber discharge could theoretically have remained altered for the observed several days. Additionally, inactivation studies of the left ventral premotor cortex demonstrate a role for this region in detecting leftward errors in reaching (Kurata and Hoshi 1999). Assuming that the left ventral premotor cortex projects (Brodal 1978; Glickstein et al. 1985) leftward error to the contralateral dentate nucleus, it remains possible that inactivation of the right cerebellar cortex might produce erroneous, excessive leftward error signals within the dentate nucleus. Signals processed in the dentate may then traverse the lower transcerebellar feedback loop (dentate nucleus to red nucleus, to inferior olive, to cerebellar cortex via the climbing fiber) to provide remaining active areas of the cerebellar cortex with a signal to compensate for the leftward error. Assuming that the posterolateral cerebellum effectively calculates and detects end point errors in reaching, the leftward error signal may produce plastic changes in other areas (such as lobule V) that control arm reaching movements (but are not inactivated by lidocaine), thus resulting in rightward errors in reaching. Such an explanation depicts a gradual increase in rightward error following inactivation of the cerebellar cortex in addition to the leftward errors provoked with inactivation of the right dentate nucleus. To better understand the delayed inactivation effects, further inactivation and recording studies would be useful in determining for how long after inactivation the CS discharge is reduced and when spontaneous discharge of the CS, Purkinje cell, and nuclear cells return to normal.
Several injections, of both control and active substances, did not result in any immediate or delayed significant effects. The lack of response to the active substances could be due to any of several uncontrolled variables (e.g., ineffective injection media, cannula targeting errors, injection into sulci, substance pooling in the inferior cerebellum, prior damage to cerebellar tissue, etc.). These explanations seem unlikely given the successful attempts using the same media and techniques that temporally flanked unsuccessful attempts, but observed cellular infiltrate on histology may account for cellular changes resulting in permanent damage to cerebellar structures.
Summary.
We conclude that inactivation of either the cerebellar cortex or the dentate nucleus causes an immediate selective impairment of prism-adjusted gaze-reach calibration. Additionally, our results suggest that disruption of these structures also contributes to delayed alteration of both prism and no-prism gaze-reach adjustments. These results do not distinguish between the two different (or combined) intracerebellar sites, and they do not exclude the possibility that the cerebellum functions to access and retrieve calibrations stored elsewhere. Nevertheless, these experiments would appear to satisfy inactivation/ablation criteria for effective localization of the gaze-reach calibration within both the cerebellar cortex and the dentate nucleus. More experiments that include both inactivation and recording techniques during the different behavioral paradigms are also needed to resolve this issue.
GRANTS
This research was funded by National Institute of Neurological Disorders and Stroke Grant NS-12777.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Tod Martin and Bradley Greger for assistance in designing the experimental paradigm and Valerie Montalvo and Steve-Felix Belinga for assistance training monkeys and conducting experiments.
Footnotes
The work by McCormick and Thompson (1984) focused on the nictitating membrane response. Often, the nictitating membrane response is described as a single muscle task but actually involves simultaneous use of other extraocular muscles beyond the retractor bulbi (see Disterhoft et al. 1985).
REFERENCES
- Anzai M, Kitazawa H, Nagao S. Effects of reversible pharmacological shutdown of cerebellar flocculus on the memory of long-term horizontal vestibulo-ocular reflex adaptation in monkeys. Neurosci Res 68: 191–198, 2010 [DOI] [PubMed] [Google Scholar]
- Arikan R, Blake NMJ, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Methods 118: 51–57, 2002 [DOI] [PubMed] [Google Scholar]
- Baizer JS, Glickstein M. Role of cerebellum in prism adaptation. J Physiol 236: 34–35, 1974 [PubMed] [Google Scholar]
- Baizer JS, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol 81: 1960–1965, 1999 [DOI] [PubMed] [Google Scholar]
- Brodal P. The corticopontine projection in the rhesus monkey: origin and principles of organization. Brain 1010: 251–283, 1978 [DOI] [PubMed] [Google Scholar]
- Darlington RB, Nathan RG. Radicals and Squares, and Other Statistical Procedures for the Behavioral Sciences. Ithaca, NY: Logan Hill, 1975 [Google Scholar]
- Della-Maggiore V, McIntosh AR. Time course of changes in brain activity and functional connectivity associated with long-term adaptation to a rotational transformation. J Neurophysiol 93: 2254–2262, 2005 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci 25: 9919–9931, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Quinn KJ, Weiss C, Shipley MT. Accessory abducens nucleus and conditioned eye retraction/nictitating membrane extension in rabbit. J Neurosci 5: 941–950, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PFC, Thach WT. Purkinje cell activity during motor learning. Brain Res 128: 309–328, 1977 [DOI] [PubMed] [Google Scholar]
- Glickstein M, May JG, II, Mercier BE. Corticopontine projection in the macaque: the distribution of labeled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol 235: 343–359, 1985 [DOI] [PubMed] [Google Scholar]
- Glickstein M. The cerebellum and motor learning. Curr Opin Neurobiol 2: 802–806, 1992 [DOI] [PubMed] [Google Scholar]
- Greger B, Norris SA, Thach WT. Spike firing in the lateral cerebellar cortex correlated with movement and motor parameters irrespective of the effector limb. J Neurophysiol 91: 576–582, 2004 [DOI] [PubMed] [Google Scholar]
- Helmholtz HLF, von Treatise on Physiological Optics, Vol. 3, Sect 29, translated from 3rd German ed by Southall JPC. Menasha, WI: George Banta, 1924–1925 [Google Scholar]
- Ito M, Shiida T, Yagi N, Yamamoto M. The cerebellar modification of rabbit's horizontal vestibulo-ocular reflex induced by sustained head rotation combined with visual stimulation. Proc Jpn Acad 50: 85–89, 1974 [Google Scholar]
- Kenyon GT, Medina JF, Mauk MD. A mathematical model of the cerebellarolivary system. I. self-regulating equilibrium of climbing fiber activity. J Comput Neurosci 5: 17–33, 1998 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci 29: 59–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science 260: 989–991, 1993 [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoshi E. Reacquisition deficits in prism adaptation after muscimol microinjection into the ventral premotor cortex of monkeys. J Neurophysiol 81: 1927–1938, 1999 [DOI] [PubMed] [Google Scholar]
- Madigan JC, Carpenter MB. Cerebellum of the Rhesus Monkey—Atlas of Lobules, Laminae, and Folia in Sections. Baltimore, MD: University Park, 1971 [Google Scholar]
- Martin JH, Cooper SE, Hacking A, Ghez C. Differential effects of cerebellar nuclei inactivation on reaching and adaptive control. J Neurophysiol 83: 1886–1899, 2000 [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119: 1183–1198, 1996a [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996b [DOI] [PubMed] [Google Scholar]
- Mauk MD. Roles of cerebellar cortex and nuclei in motor learning: contradictions or clues? Neuron 18: 343–346, 1997 [DOI] [PubMed] [Google Scholar]
- Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn Mem 4: 130–158, 1997 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned nictitating membrane response. Science 223: 296–299, 1984 [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Takada M, Hattori T. Morphological changes of striatal dopaminergic presynaptic boutons following intrastriatal kainate injection. Neuropharmacology 30: 813–817, 1991 [DOI] [PubMed] [Google Scholar]
- Mugnaini E. The length of cerebellar parallel fibers in chicken and rhesus monkey. J Comp Neurol 220: 7–15, 1983 [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitazawa H. Effects of reversible shutdown of the monkey flocculus on the retention of adaptation of the horizontal vestibulo-ocular reflex. Neuroscience 118: 563–570, 2003 [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitazawa H. Role of the cerebellum in the acquisition and consolidation of motor memory. Brain Nerve 60: 783–790, 2008 [PubMed] [Google Scholar]
- Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res 140: 66–76, 2001 [DOI] [PubMed] [Google Scholar]
- Norris SA, Greger B, Hathaway EN, Thach WT. Purkinje cell spike firing in the posterolateral cerebellum: correlation with visual stimulus, oculomotor response, and error feedback. J Neurophysiol 92: 1867–1879, 2004 [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Mauk MD. Stimulus generalization of conditioned eyelid responses produced without cerebellar cortex: implications for plasticity in the cerebellar nuclei. Learn Mem 10: 346–354, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci 13: 1708–1718, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci 1: 153–170, 1989 [DOI] [PubMed] [Google Scholar]
- Pisella L, Rossetti Y, Michel C, Rode G, Boisson D, Pelisson D, Tilikete C. Ipsidirectional impairment of prism adaptation after unilateral lesion of anterior cerebellum. Neurology 65: 150–152, 2005 [DOI] [PubMed] [Google Scholar]
- Porrill J, Dean P. Cerebellar motor learning: when is cortical plasticity not enough? PLoS Comput Biol 3: 1935–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during non-motor learning. Cereb Cortex 4: 8–26, 1994 [DOI] [PubMed] [Google Scholar]
- Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev 29: 431–444, 2005 [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 5: 813–819, 2004 [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res 68: 168–178, 1987 [DOI] [PubMed] [Google Scholar]
- Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience 5: 991–1014, 1980 [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophysiol 99: 1836–1845, 2008 [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res 175: 544–555, 2006 [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage 22: 1775–1783, 2004 [DOI] [PubMed] [Google Scholar]
- Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience 139: 767–777, 2006 [DOI] [PubMed] [Google Scholar]
- Snider RS, Lee JC. A Stereotaxic Atlas of the Monkey Brain (Macaca mulatta). Chicago, IL: Univ. of Chicago Press, 1961 [Google Scholar]
- Soteropoulos DS, Baker SN. Bilateral representation in the deep cerebellar nuclei. J Physiol 586: 1117–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT. Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol 30: 675–696, 1967 [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol 31: 785–797, 1968 [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HG, Keating JG. Cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15: 403–442, 1992 [DOI] [PubMed] [Google Scholar]
- Thach WT, Kane SA, Goodkin HP. Dentate/interposed cerebellar nuclear sites coordinating reach and grasp. Abstr Soc Neurosci 24: 1406, 1998 [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33: 766–772, 1983 [DOI] [PubMed] [Google Scholar]
- Werner S, Bock O, Gizewski ER, Schoch B, Timmann D. Visuomotor adaptive improvement and aftereffects are impaired differentially following cerebellar lesions in SCA and PICA territory. Exp Brain Res 201: 429–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters WD, Kado RT, Adey WR. A Stereotaxic Brain Atlas for Macaca nemestrina. Berkeley, CA: Univ. of California Press, 1969 [Google Scholar]
- Yeo CH, Hardman MJ, Glickstein M. Classical condition of the nictitating membrane response of the rabbit II. Lesions of the cerebellar cortex. Exp Brain Res 60: 99–113, 1985 [DOI] [PubMed] [Google Scholar]