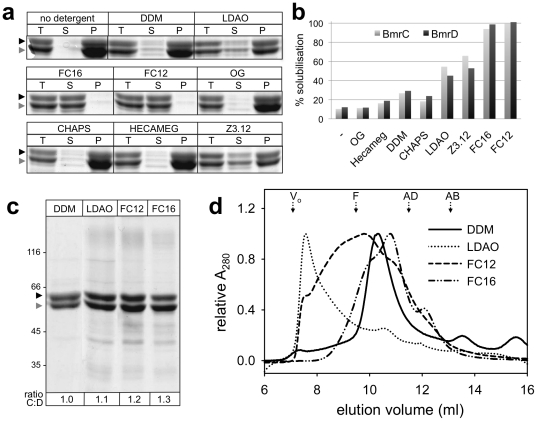
Figure 1. Purification of BmrC/BmrD using different detergents.
(a) BmrC/BmrD solubilization by non-ionic and zwitterionic detergents was performed at 2 mg/ml protein and 10 mg/ml detergent and assessed by Coomassie Blue stained SDS-PAGE of the soluble (S) and insoluble (P) material obtained from 15 µg of total protein sample (T). Note that the strong densities observed in some of the pellet bands probably arise from difficulties encountered while resuspending the pellet that led to inhomogeneous samples. Black arrowhead, BmrD; grey arrowhead, BmrC. (b) The intensities of the bands corresponding to the S and T samples were quantitated using Image Gauge software (Fuji Film Science Lab) and the solubilization yields were calculated as the ratio of each protein in the supernatant (S) relative to its amount in the membrane fraction (T). (c, d) Protein purified by nickel affinity chromatography in the presence of the indicated detergents was analyzed by (c) Coomassie Blue stained SDS-PAGE (5 µg per lane, except for DDM : 4 µg) and (d) size-exclusion chromatography performed on a Superdex 200 10/300 GL column using 0.05% (w/v) DDM in the elution buffer. Elution volumes of proteins used for column calibration are indicated above: ferritin (F, 440 kDa), aldolase (AD, 158 kDa), albumin (AB, 67 kDa), Vo: void volume. The intensities of the BmrC and BmrD bands in the polyacrylamide gel in c were quantitated and the ratio BmrC∶BmrD (ratio C∶D) was indicated under each lane. Black arrowhead, BmrD; grey arrowhead, BmrC.