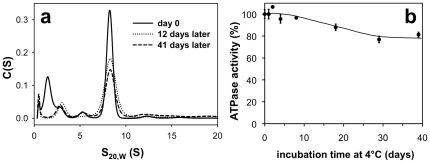
Figure 3. Stability of DDM-purified BmrC/BmrD.
(a) After nickel-affinity and size-exclusion chromatography in the presence of DDM, purified BmrC/BmrD protein at 0.11 mg/ml was immediately subjected (day 0) to sedimentation velocity measurements or incubated at 4°C for 12 or 41 days before performing analytical ultracentrifugation experiments at 42000 rpm and 20°C. The superposition of the c(s) curves from data obtained at 278 nm at different times is shown. (b) ATPase activity of nickel-affinity purified BmrC/BmrD was determined in 0.05% (w/v) DDM after incubation at 4°C for the indicated times. The values are expressed as the mean ± SD for three measurements, relative to the ATPase activity obtained without prior incubation at 4°C (244±10 nmol·min−1·mg−1).