Table 1.
ddition of Phenols to Activated Allenesa
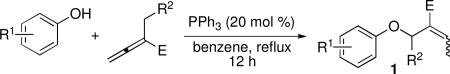 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | E | 1 | yield (%)b | E:Zc |
| 1 | H | H | CO2Et | 1a | >99 | E only |
| 2 | 4-Me | H | CO2Et | 1b | 97 | E only |
| 3 | 4-OMe | H | CO2Et | 1c | 92 | E only |
| 4 | 4-I | H | CO2Et | 1d | 69 | E only |
| 5 | 4-Br | H | CO2Et | 1e | 72 | E only |
| 6 | 4-Cl | H | CO2Et | 1f | 62 | E only |
| 7 | 4-F | H | CO2Et | 1g | 78 | E only |
| 8 | 4-CF3 | H | CO2Et | 1h | 73 | E only |
| 9 | 4-CO2Et | H | CO2Et | 1i | 90 | E only |
| 10 | 4-NHBoc | H | CO2Et | 1j | 89 | 4:1 |
| 11 | 3-Me | H | CO2Et | 1k | 95 | E only |
| 12 | 2-Me | H | CO2Et | 1l | 96 | 20:1 |
| 13 | 2-I | H | CO2Et | 1m | 91 | E only |
| 14 | 2,6-di-Me | H | CO2Et | 1n | 66 | E only |
| 15 | 2,6-di-t-Bu | H | CO2Et | 1o | 0 | N/A |
| 16 | 4-OMe | Ph | CO2Et | 1p | 89 | Z only |
| 17 | 4-CF3 | Ph | CO2Et | 1q | 97 | Z only |
| 18 | 4-CF3 | Ph | CN | 1r | 88 | Z only |
Reactions were performed using 1.1 equiv of allene.
Isolated yield.
Determined through 1H NMR spectroscopic analysis.
