Table 2.
Addition of Amines to α-Methyl Allenoatea
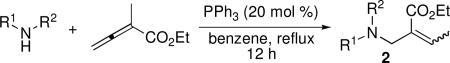 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | yield (%)b | 2 | E:Zc |
| 1 | Ph | p-Ts | 94 | 2a | 2.5:1 |
| 2 | Bn | p-Ts | 94 | 2b | 2.5:1 |
| 3 | Boc | p-Ts | 83 | 2c | 2:1 |
| 4 | H | p-Ts | 53 | 2d | 1:1.75 |
| 5 | Ph | Boc | 0 | 2e | N/A |
| 6 | phthalimide | 71 | 2f | E only | |
| 7 | TsHN–NH2 | 57 | 2g | 1.5:1 | |
| 8 |
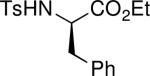
|
92 | 2h | 1:1.2 | |
Reactions were performed using 1.1 equiv of the allenoate.
Isolated yield.
Determined through 1H NMR spectroscopic analysis.
