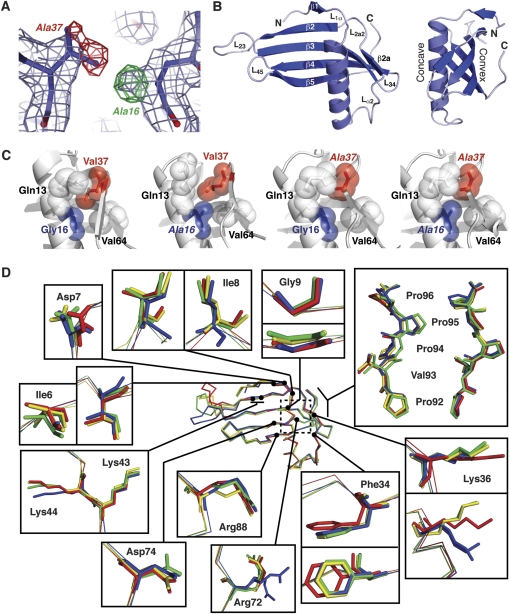
Figure 1.
Crystal structures of wild-type and mutant MNEI proteins. (A) Example of electron density quality at the mutated residues. The wild-type structure (G16/V37) is shown in 2Fo - Fc density (light blue) and Fo - Fc difference density (positive and negative shown in green and red, respectively) generated using the wild-type model and G16A/V37A-MNEI X-ray data. Changes required in the mutant model are indicated next to each peak in the difference map. (B) Cartoon of the WT-MNEI X-ray crystal structure shown in 2 views related by a 90° rotation about the vertical axis. Protein termini, β-strands and loops, including the engineered loop L23, are indicated. Amino acids 16 and 37 are located on the α-helix and strand β2a, respectively. (C) Organization of the MNEI protein core surrounding the mutated positions 16 and 37. Sidechains are shown with van der Waal's radii (semitransparent spheres) with mutated residues indicated with italic font. (D) Backbone alignment of WT- (blue), G16A- (red), V37A- (gold), and G16A/V37A-MNEI (green) with zoomed views of key surface residues for monellin sweetness. The dashed box indicates the protein core containing the mutated residues, shown in panel (C).