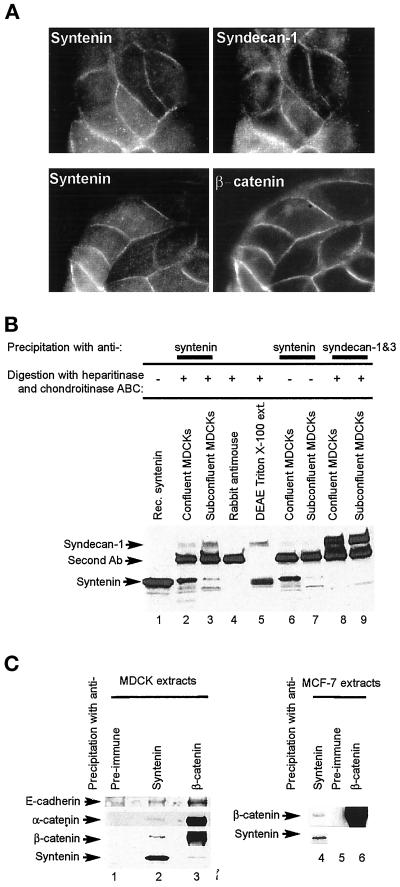
Figure 7.
Syndecan-1 and the E-cadherin/β-catenin/α-catenin complex colocalize in cell-cell contacts and coimmunoprecipitate with syntenin from epithelial cell extracts. (A) Colocalization of syntenin with syndecan-1 and β-catenin. Fluorescence optical micrographs of MDCK cells stained with affinity-purified rabbit anti-syntenin polyclonal antibodies (left) and costained with mouse mAbs for syndecan-1 and 3 (upper right) or β-catenin (lower right). (B) Coimmunoprecipitation of syndecan-1 with syntenin. Cross-linked protein extracts from confluent (lanes 2, 4, 6, and 8) or subconfluent (lanes 3, 7, and 9) MDCK cells were immunoprecipitated with mouse anti-syntenin (lanes 2, 3, 6, and 7) or anti-syndecan (lanes 8 and 9) mAbs and rabbit antimouse polyclonal antibodies (lanes 2–4 and 6–9). In vitro transcribed-translated syntenin (lane 1), proteoglycans purified from Triton X-100 extracts of MDCK cells by chromatography over DEAE (lane 5), and the immunoprecipitated proteins were digested (lanes 2–5, 8, and 9) or not digested (lanes 6 and 7) with heparitinase and chondroitinase ABC. Samples were Western blotted for syndecan-1 and -3 (upper part) or syntenin (lower part). The band specifically stained with the 2E9 anti-syndecan antibody migrates at a position that is characteristic for syndecan-1, which is consistent with the fact that syndecan-1 is, and syndecan-3 is not expressed in MDCK cells. (C) Syntenin and members of the E-cadherin adhesion complex coimmunoprecipitate. Noncross-linked protein extracts from MDCK (lanes 1–3) or MCF-7 (lanes 4–6) cells were immunoprecipitated with preimmune serum (lanes 1 and 5), affinity-purified anti-syntenin polyclonal (lanes 2 and 4), or anti-β-catenin serum (lanes 3 and 6) and Western blotted for the proteins indicated.