Figure 5.
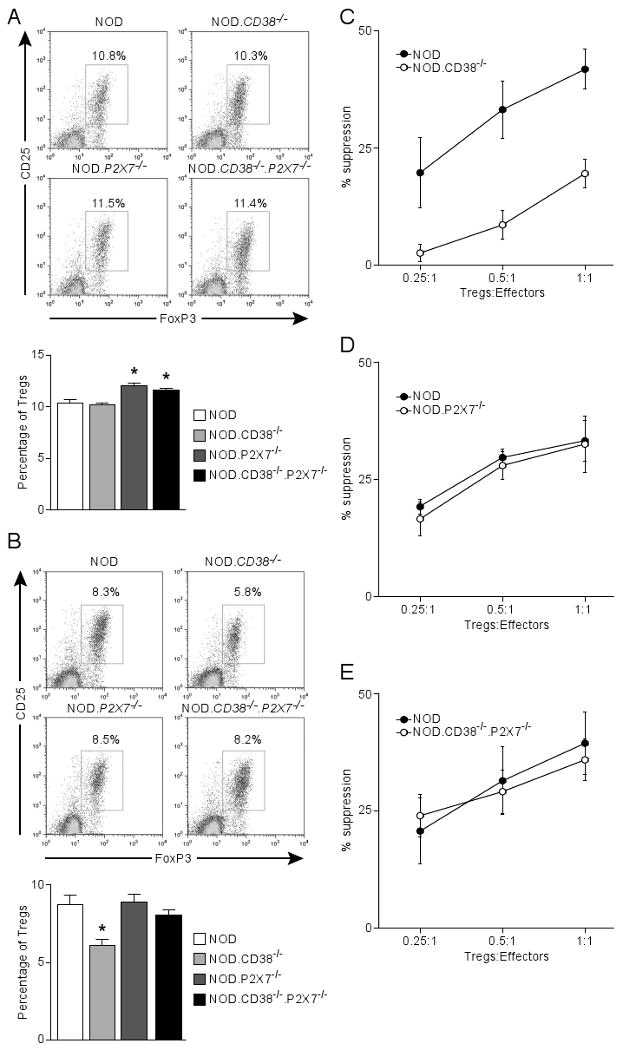
Co-ablation of P2X7 improves the survival and function of Tregs in CD38-deficient NOD mice. The frequency of CD4 Tregs (CD25+Foxp3+) was determined in the spleens (A) and PLN (B) of 10-11 week-old male NOD mice and those deficient in CD38 and/or P2X7. (A) The upper panel shows the representative CD25 and Foxp3 staining (gated on CD4 T cells). Results are summarized in the lower panel. *P<0.05 compared to NOD mice (Mann-Whitney test, n=5-6 per genotype). (B) The upper panel shows the representative CD25 and Foxp3 staining (gated on CD4 T cells). Results are summarized in the lower panel. *P<0.05 compared to NOD mice (Mann-Whitney test, n=5-6 per genotype). (C, D, E) In vitro Treg assay comparing the suppressive activity between indicated strains (age-matched females at 6-8 weeks). NOD effector T-cells (CD4+CD25-) were labeled with CFSE and co-cultured at indicated ratios with Tregs (CD4+CD25+) in triplicate in a 96-well plate in the presence of NOD.scid splenocytes (2×105) and 5 μg/ml anti-CD3 for 3 days. Proliferation of effector T-cells was determined by CFSE dilution. The percentage of suppression is defined by the reduction in the proportion of divided effector T-cells relative to that of the control without Tregs. Results indicate the mean±sem of 3 independent experiments.
