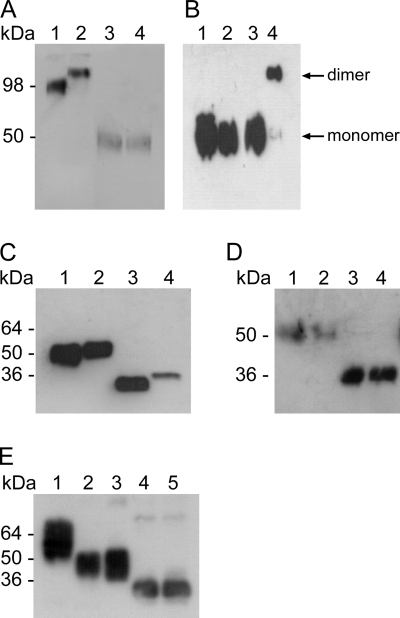
Fig. 2.
Structure and biochemical properties of ssGP. (A) ssGP is a homodimer. Vero E6 cells were transfected with the appropriate expression plasmids, and supernatants were collected from transfected cells after 72 h. The proteins were separated by reducing or nonreducing SDS-PAGE and were detected by immunoblotting using MAb 42/3.7 (dilution, 1:10,000). Lane 1, r.ssGP (nonreducing); lane 2; r.sGP (nonreducing); lane 3, r.ssGP (reducing); lane 4, r.sGP (reducing). (B) Cysteine at position 53 is responsible for dimerization. Site-directed mutagenesis was performed at amino acid position 53 (cysteine to glycine), and the resulting plasmid was transfected into 293T cells. Proteins were detected by immunoblotting with a peroxidase-conjugated anti-HA antibody (dilution, 1:10,000). Lane 1, r.ssGP Cys53Gly mutant (reducing); lane 2, r.ssGP (reducing); lane 3, r.ssGP Cys53Gly mutant (nonreducing); lane 4, r.ssGP (nonreducing). (C) ssGP contains N-linked carbohydrates. Vero E6 cells were transfected with the appropriate expression plasmids, and supernatants were collected from transfected cells after 72 h. The proteins were treated with PNGase F, separated by reducing SDS-PAGE, and detected by immunoblotting using MAb 42/3.7 (dilution, 1:10,000). Lane 1, untreated r.ssGP; lane 2, untreated r.sGP; lane 3, PNGase F-treated r.ssGP; lane 4, PNGase F-treated r.sGP. (D) ssGP does not contain high-mannose-type N-linked carbohydrates. 293T cells were transfected with the appropriate plasmid expressing HA-tagged r.ssGP, and HA-tagged r.ssGP was purified from the supernatants 72 h posttransfection (see Materials and Methods). HA-tagged r.ssGP was treated with Endo H and/or PNGase F and was analyzed by reducing SDS-PAGE followed by immunoblotting using peroxidase-conjugated anti-HA (dilution, 1:10,000). Lane 1, untreated r.ssGP; lane 2, Endo H-treated r.ssGP; lane 3, r.ssGP treated with Endo H and PNGase F; lane 4, r.ssGP treated with PNGase F. (E) ssGP does not contain O-linked carbohydrates. HA-tagged r.ssGP treated with different exoglycosidases, O-glycanase, N-glycanase, or combinations of these glycosidases was analyzed as described in Materials and Methods. Lane 1, untreated r.ssGP; lane 2, r.ssGP treated with the exoglycosidases [sialidase A, β(1–4)-galactosidase, and β-N-acetylglucosaminidase]; lane 3, r.ssGP treated with the exoglycosidases and O-glycanase; lane 4, r.ssGP treated with the exoglycosidases, O-glycanase, and N-glycanase; lane 5, r.ssGP treated with N-glycanase.