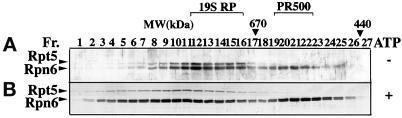
Figure 1.
Rpn6 is present in two distinct protein complexes in plants. Cell extract from cauliflower head was fractionated in a Superose 6 gel filtration column. Selected elution fractions (Fr.) of 0.25 ml each were analyzed by immunoblot for Rpn6 and Rpt5. The elution positions of molecular mass markers are shown (in kDa) above the gel blots. (A) Cell extraction and subsequent size fractionation were conducted under standard condition (see MATERIALS AND METHODS) in which no ATP was present. (B) ATP was included in the extraction buffer, the column equilibration buffer, and elution buffer in otherwise identical experimental conditions as in A. Note that Rpn6 is eluted in a peak at ∼500 kDa in the presence and absence of ATP and that both Rpn6 and Rpt5 cofractionate in an 800-kDa peak in the absence of ATP and in fractions of larger molecular mass in the presence of ATP.