Abstract
We sought to define the risk of neonatal respiratory distress syndrome (RDS) as a function of both lecithin/sphingomyelin (L/S) ratio and gestational age. Amniotic fluid L/S ratio data were collected from consecutive women undergoing amniocentesis for fetal lung maturity at Yale-New Haven Hospital from January 1998 to December 2004. Women were included in the study if they delivered a live-born, singleton, nonanomalous infant within 72 hours of amniocentesis. The probability of RDS was modeled using multivariate logistic regression with L/S ratio and gestational age as predictors. A total of 210 mother-neonate pairs (8 RDS, 202 non-RDS) met criteria for analysis. Both gestational age and L/S ratio were independent predictors of RDS. A probability of RDS of 3% or less was noted at an L/S ratio cutoff of ≥3.4 at 34 weeks, ≥2.6 at 36 weeks, ≥1.6 at 38 weeks, and ≥1.2 at term. Under 34 weeks of gestation, the prevalence of RDS was so high that a probability of 3% or less was not observed by this model. These data describe a means of stratifying the probability of neonatal RDS using both gestational age and the L/S ratio and may aid in clinical decision making concerning the timing of delivery.
Keywords: Respiratory distress syndrome, lecithin/sphingomyelin ratio, fetal lung maturity
Neonatal respiratory distress syndrome (RDS) refers to respiratory compromise presenting at or shortly after delivery due specifically to a deficiency of pulmonary surfactant, a naturally occurring phospholipid detergent required to decrease surface tension within the alveoli thereby preventing alveolar collapse. Originally described by Avery and Mead in 1959,1 RDS remains a major cause of neonatal morbidity and mortality. Neonatal RDS affects ~1% of all live births2–4; however, not all infants are at equal risk. The pulmonary system is among the last of the fetal organ systems to become functionally mature. As such, RDS is primarily—although not exclusively—a disease of premature infants with an incidence and severity that is highly dependent upon gestational age.2–6
Given the gravity of neonatal RDS, several biochemical tests have been developed to predict the risk of RDS and thereby assist obstetric care providers in timing delivery in high-risk pregnancies. All of these tests require amniocentesis with direct or indirect measurement of the surface-active properties of surfactant phospholipids secreted by the fetal lungs into the amniotic fluid. Such tests include, among others, the lecithin/sphingomyelin (L/S) ratio, foam stability index, surfactant-to-albumin ratio, lamellar body count, and presence or absence of phosphatidylglycerol (PG). The thin-layer chromatography L/S ratio is one of the oldest, best validated, and most widely used of these tests. It is based on the original observations by Gluck and colleagues7,8 that lecithin (but not sphingomyelin) in the fetal pulmonary secretions increases after 32 to 33 weeks of gestation and that neonatal RDS was uncommon (2.6%) after the L/S ratio reached 2.0, which occurs around 35 weeks in uncomplicated pregnancies. This cutoff has remained an accepted threshold for the determination of fetal pulmonary maturity in nondiabetic pregnancies regardless of gestational age.
Despite the well-known relationship between RDS and gestational age,2–6 biochemical tests for fetal lung maturity have traditionally been interpreted as dichotomous (either positive or negative) without regard for gestational age. This study was therefore designed to derive predictive logistic regression equations to allow the risk of neonatal RDS to be defined as a function of both the L/S ratio and gestational age.
MATERIALS AND METHODS
Study Population
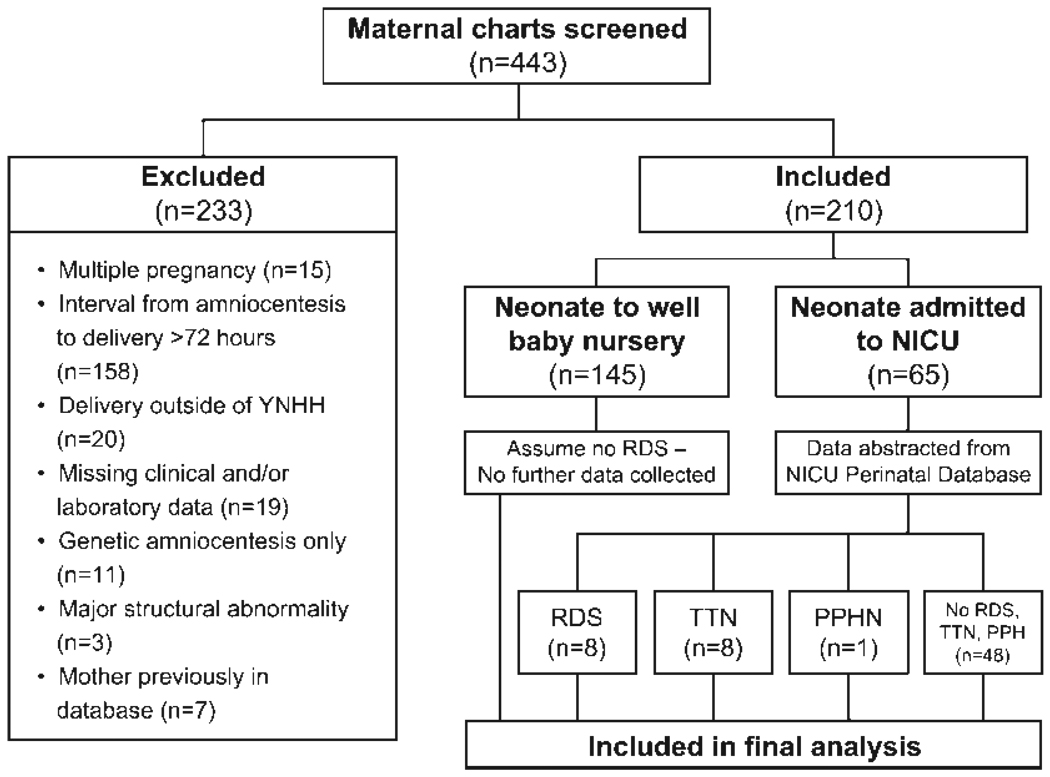
Consecutive singleton, nonanomalous, live-born infants born to women who underwent amniocentesis for fetal lung maturity screening with the L/S ratio at Yale-New Haven Hospital from January 1998 to December 2004 were identified. The study was approved by the hospital’s Human Investigations Committee. Patients were included in the study if L/S ratio measurements were obtained within 72 hours of delivery. Maternal records were matched to neonatal charts and demographic and outcome data abstracted. Maternal charts were reviewed for, among other data, gestational dating criteria, indication and results of L/S ratio and PG testing, date of delivery, maternal ethnicity, antenatal corticosteroid administration, smoking status, and the presence of medical complications, including diabetes and hypertensive disorders of pregnancy. Neonatal records provided information on infant gender, birth weight, Apgar scores, results of chest radiographs, and newborn intensive care unit (NICU) course. Subjects were excluded if the interval from amniocentesis to delivery was >72 hours, if there were major congenital anomalies, if amniocentesis was performed for karyotype analysis only (no L/S ratio was measured), or if there were missing clinical and/or laboratory data (Fig. 1). If women had an amniocentesis for fetal lung maturity in more than one pregnancy, only the first pregnancy was included to maintain independence of our data points.
Figure 1.
The method of subject selection resulting in the study population is shown. NICU, neonatal intensive care unit; PPHN, persistent pulmonary hypertension; RDS, respiratory distress syndrome; TTN, transient tachypnea of the newborn; YNHH, Yale-New Haven Hospital.
Gestational age was determined by first-trimester ultrasound, a firm last menstrual period confirmed by a second-trimester ultrasound, or in vitro fertilization or artificial insemination dating, as available. All subjects met these criteria. Maternal race was self-described in the medical record. Maternal diabetes was defined as any insulin requirement, including insulin-dependent pre-gestational diabetes and insulin-requiring gestational diabetes. Preeclampsia was defined as the presence of new-onset hypertension (sitting blood pressure ≥140 mm Hg systolic and/or ≥90 mm Hg diastolic) and new-onset proteinuria (≥300 mg per 24 hours) after 20 weeks’ gestation. Chronic hypertension was defined by maternal antihypertensive use before pregnancy. Maternal smoking status was identified in the medical record. Antenatal corticosteroid status was defined as a complete 48-hour course of steroids.
Amniotic fluid specimens were analyzed by thin-layer chromatography as previously described7,8 and the L/S ratio calculated. The presence or absence of a PG band was also noted on the gel. Because this was a clinically indicated test, results of the L/S ratio and PG tests were available to the obstetric care provider and were used in clinical decision making. At our institution, the L/S ratio cutoff to predict pulmonary maturity is 2.5 for nondiabetics and 3.0 for diabetics.
After delivery, infants who remained in the well-baby nursery were assumed not to have RDS. For those infants admitted to the NICU, data were collected on the diagnoses of RDS, transient tachypnea of the newborn (TTN), persistent pulmonary hypertension (PPHN), bronchopulmonary dysplasia, pneumothorax, and other neonatal complications. Neonatal RDS was diagnosed by the presence of at least two of the following three criteria: (1) evidence of respiratory compromise (tachypnea, retractions, and/or nasal flaring) shortly after delivery and a persistent oxygen requirement for longer than 24 hours, (2) administration of exogenous pulmonary surfactant, and/or (3) radiographic evidence of hyaline membrane disease.
Statistical Analysis
The data were analyzed using the SAS 9.1 statistical software package. Maternal and neonatal characteristics were compared in infants with and without RDS using chi-square analysis and Fisher exact test. Gestational age at the time of amniocentesis was analyzed using the Student t test; these values were compared in neonates with and without RDS by Pooled and Satterthwaite t tests after assessment of the equality of variances. Wald odds ratios (ORs) with 95% confidence intervals (95% CI) were determined for predictors of neonatal RDS and after adjusting for gestational age. Finally, using multivariate logistic regression, a prediction equation for the probability of neonatal RDS was developed with both the L/S ratio value and gestational age as the primary descriptive variables. Stepwise backward elimination was used to eliminate possible confounders, and the data presented as probability of neonatal RDS by gestational age and L/S ratio values. This equation is discussed later in this article.
RESULTS
Study Population
A total of 443 matched maternal-neonatal records were identified and screened for this study. Of these, 233 were excluded (Fig. 1) leaving 210 mother-neonate pairs who met criteria for analysis. The most common cause for exclusion (158/233 [68%]) was a time interval from amniocentesis to delivery of >72 hours. Seven maternal-neonate pairs (3%) were excluded because the mother was already represented in the database. Three cases (1%) were excluded due to severe congenital malformations that could complicate the diagnosis of neonatal RDS, primarily congenital cystic adenomatous malformation.
Table 1 presents the maternal and neonatal clinical characteristics of the study population and their association with neonatal RDS. Within the limits of our sample size, the clinical characteristics of maternal age, race, tobacco use, diabetes, and presence or absence of hypertension and preeclampsia did not differ among maternal-infant dyads who did and did not develop RDS (Table 1). Forty of the 210 mother-neonate pairs (19.1%) received antenatal corticosteroids. In the population without RDS, 16.3% (33/202) received steroids as compared with 87.5% (7/8) of the RDS population (p < 0.0001). As the benefit of antenatal corticosteroids in preventing neonatal RDS after 34 weeks is unproven9,10 and the average gestational age in the study population was 36 weeks, this difference may serve as a marker of lower gestational age in the RDS population.
Table 1.
Maternal and Neonatal Characteristics by RDS Status
| RDS Status | |||
|---|---|---|---|
| Characteristic | No RDS (n = 202) |
RDS (n = 8) |
p Value |
| Maternal age (y) | 31.0 ± 6.0 | 30.0 ± 9.9 | 0.78 |
| Race | |||
| White | 118 (58.4%) | 3 (37.5%) | 0.23 |
| Black | 36 (17.8%) | 1 (12.5%) | |
| Hispanic | 26 (12.9%) | 3 (37.5%) | |
| Asian/South Asian | 7 (3.5%) | 0 (0%) | |
| Other/not documented | 15 (7.4%) | 1 (12.5%) | |
| Tobacco use | 29 (14.4%) | 0 (0%) | 0.60 |
| Diabetes | |||
| No diabetes | 135 (66.8%) | 8 (100%) | 0.23 |
| Diet-controlled diabetes | 20 (9.9%) | 0 (0%) | |
| Insulin-requiring diabetes | 47 (23.3%) | 0 (0%) | |
| Chronic hypertension | 26 (12.9%) | 0 (0%) | 0.60 |
| Preeclampsia | 14 (6.9%) | 0 (0%) | 0.57 |
| Preterm PROM | 3 (1.5%) | 1 (12.5%) | 0.14 |
| Neonatal gender | |||
| Male | 102 (50.5%) | 3 (37.5%) | 0.72 |
| Female | 100 (49.5%) | 5 (62.5%) | |
| Antenatal steroids | 33 (16.3%) | 7 (87.5%) | <0.0001 |
| L/S ratio | 3.6 ± 1.1 | 1.8 ± 0.7 | <0.0001 |
| Gestational age at the time of amniocentesis (wk) | 36.5 ± 1.2 | 32.6 ± 3.6 | <0.0001 |
Values are expressed as n (%) or mean ± standard deviation.
p values were calculated using t test, chi-square, or Fisher exact test.
L/S, lecithin/sphingomyelin; PROM, premature rupture of membranes; RDS, respiratory distress syndrome.
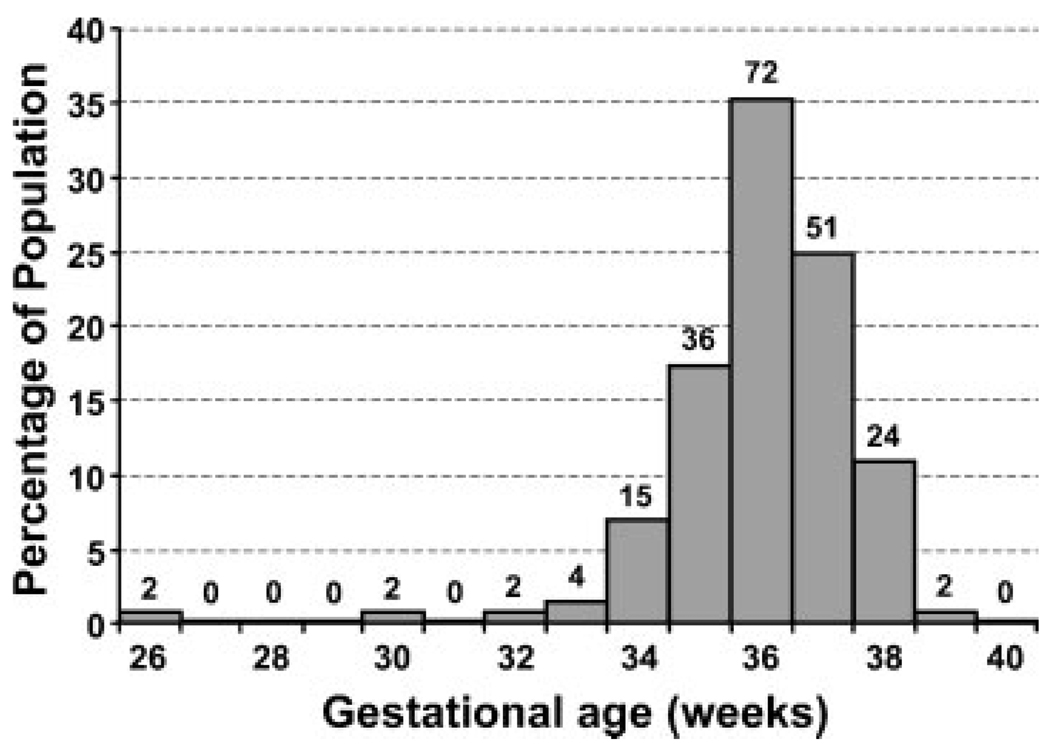
Of the 210 infants in the final analysis, 145 (69%) remained in the well-baby nursery and 65 (31%) were admitted to the NICU. Those infants who remained in the well-baby nursery were assumed not to have RDS. Of the 210 infants, there were eight cases of neonatal RDS for an incidence of 3.8%. An additional eight neonates (3.8%) were noted to have TTN and one newborn (0.05%) was diagnosed with PPHN. The gestational age distribution of the patient population at the time of amniocentesis is shown in Fig. 2. In the population without RDS (n = 202), the mean (± standard deviation) gestational age was 36.5 ± 1.2 weeks (range 32.9 to 39.1). In the population with RDS (n = 8), the mean gestational age was 32.6 ± 3.6 weeks (range 26.0 to 37.4). The difference in the mean gestational age between the two populations was statistically significant (p = 0.02).
Figure 2.
Gestational age distribution of the patient population. Data represents gestational age at amniocentesis; all lecithin/sphingomyelin ratio values included in the final analysis were obtained within 72 hours of delivery. Absolute patient numbers are included above each bar.
Incidence of RDS
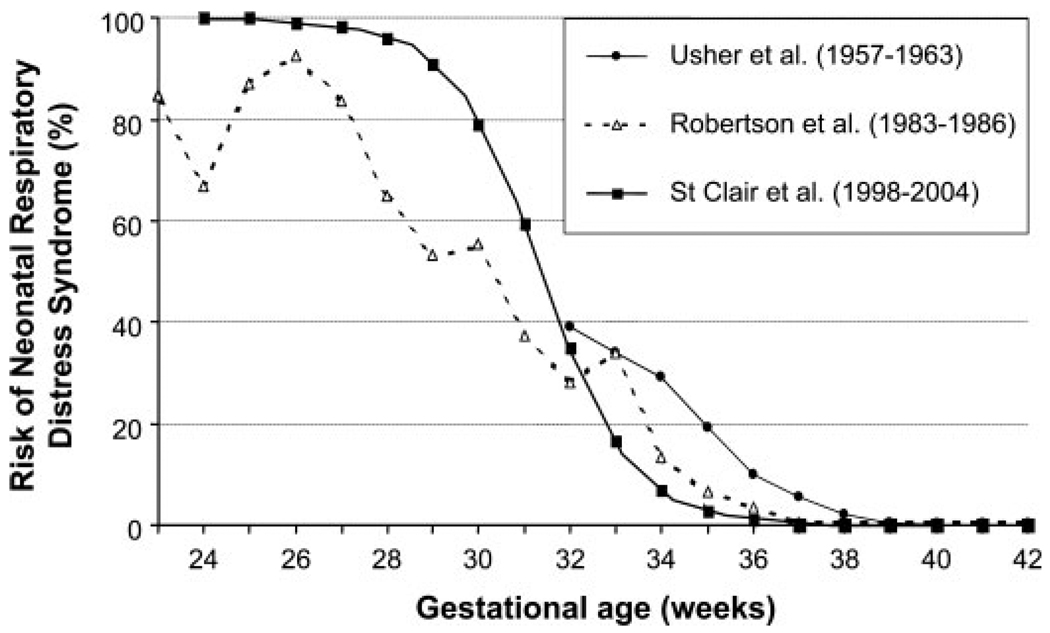
The incidence of RDS declined with increasing gestational age. Although the overall incidence of RDS in the study population was 3.8% (8/210), the incidence of RDS was 80% (4/5) for delivery < 32.9 weeks, 2.4% (3/127) at 33.0 to 36.9 weeks, and 1.3% (1/78) for delivery ≥37 weeks. A univariate logistic regression equation was derived using this data, and the probability of neonatal RDS for each week of gestational age determined (Fig. 3).
Figure 3.
Risk of neonatal respiratory distress syndrome (RDS) as a function of gestational age. Data are shown for the current population and from two prior publications showing the change in prevalence of RDS over time between 32 and 38 weeks likely due to the introduction of antenatal corticosteroids: Usher et al9 from 1957–1963 (prior to the introduction of antenatal steroids) and Robertson et al3 from 1983–1986 (where ~;40% of subjects received antenatal steroids).
L/S Ratio and Prediction of RDS
In the subjects without RDS, the mean (± standard deviation) L/S ratio was 3.6 ± 1.1 (range 1.4 to 8.5). In the subjects with RDS, the mean L/S ratio was 1.8 ± 0.7 (range 1.0 to 2.8). The difference in L/S ratio between the two groups was statistically significant (p < 0.0001).
ORs were calculated for the variables of interest (namely, gestational age and L/S ratio) as well as other commonly cited predictors of neonatal RDS (Table 2). Consistent with prior publications, both gestational age and L/S ratio were significant predictors of RDS with crude (unadjusted) ORs of 0.40 (95% CI, 0.21 to 0.75) and 0.08 (95% CI, 0.02 to 0.42), respectively. When adjusted for gestational age, the L/S value remained significant with an OR of 0.16 (95% CI, 0.03 to 0.86; p = 0.03). Thus, each 1-unit increase in the L/S ratio conferred an 84% decrease in the risk of RDS. The crude OR for receiving antenatal steroids reveals a significant association with RDS (OR, 21.5; 95% CI, 2.43 to 190.3; p = 0.005). However, once properly adjusted for gestational age at delivery, this association becomes non-significant (OR, 6.83; 95% CI, 0.64 to 72.5; p = 0.11) (Table 2). The presence of PG in the amniotic fluid also appeared to predict a significant decrease in the risk of neonatal RDS (crude OR, 0.05; 95% CI, 0.01 to 0.42; p = 0.006), but this effect disappeared after adjustment for gestational age (OR, 0.11; 95% CI, 0.01 to 1.14; p = 0.06). When the PG status was analyzed with the L/S ratio, only the L/S ratio was found to be predictive of RDS (data not shown). Finally, African-American race, neonatal gender, and preterm premature rupture of membranes were not predictive of neonatal RDS (Table 2). It was not possible to calculate ORs for maternal tobacco use, preeclampsia, and diabetes in predicting RDS given the small number of cases in our study population.
Table 2.
Likelihood of Neonatal Respiratory Distress Syndrome
| OR | 95% CI | Adjusted OR* | 95% CI | |
|---|---|---|---|---|
| Gestational age (wk)† | 0.40 | 0.21–0.75 | — | — |
| L/S ratio‡ | 0.08 | 0.02–0.42 | 0.16 | 0.03–0.86 |
| PG band | 0.05 | 0.01–0.42 | 0.11 | 0.01–1.14 |
| Antenatal steroids | 21.50 | 2.43–190.3 | 6.83 | 0.64–72.5 |
| African-American race‡ | 0.60 | 0.07–5.37 | 0.87 | 0.08–9.45 |
| Male infant | 0.62 | 0.14–2.85 | 1.87 | 0.25–13.8 |
| Preterm PROM | 3.93 | 0.31–49.1 | 0.45 | 0.01–16.9 |
Adjusted for gestational age.
Change in risk for each 1 week increment in gestational age or 1 unit increase in L/S ratio.
Compared with all other races.
OR, odds ratio; CI, confidence interval; L/S, lecithin/sphingomyelin; PG, phosphatidylglycerol; PROM, premature rupture of membranes.
Using this study population, predictive equations were developed showing the probability of neonatal RDS by gestational age and L/S ratio. Equation 2 shows Eq. 1 rearranged to solve for the probability of RDS:
| (1) |
| (2) |
where GA = gestational age.
Table 3 displays the L/S ratio cutoffs with the corresponding risk of neonatal RDS for each week of gestation after 26 weeks. Bold values in Table 3 represent a probability of neonatal RDS closest to <3%, the current risk of RDS associated with an L/S ratio of 2.0 in the literature.7,8 In this region of risk, this table illustrates that for every week below 39 weeks the L/S ratio must be 0.4 to 0.6 units higher to achieve the same risk of RDS.
Table 3.
Predicted Probability of Neonatal Respiratory Distress Syndrome by Gestational Age and Lecithin/Sphingomyelin Ratio
| Gestational age (wk) |
Lecithin/Sphingomyelin Ratio | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 1.2 | 1.2 | 1.6 | 1.8 | 2.0 | 2.2 | 2.4 | 2.6 | 2.8 | 3.0 | 3.2 | 3.4 | 3.6 | |
| <26 | 1.00 | 1.00 | 1.00 | 1.00 | .999 | .999 | .999 | .998 | .997 | .996 | .993 | .990 | .985 | .977 |
| 27 | 1.00 | 1.00 | .999 | .999 | .999 | .998 | .997 | .995 | .992 | .988 | .983 | .974 | .961 | .942 |
| 28 | .999 | .999 | .998 | .997 | .996 | .994 | .991 | .987 | .980 | .970 | .956 | .934 | .904 | .861 |
| 29 | .998 | .997 | .996 | .993 | .990 | .985 | .977 | .966 | .950 | .926 | .892 | .845 | .783 | .704 |
| 30 | .995 | .992 | .989 | .983 | .974 | .962 | .943 | .916 | .878 | .827 | .759 | .676 | .579 | .476 |
| 31 | .987 | .980 | .971 | .956 | .935 | .905 | .863 | .807 | .734 | .646 | .547 | .443 | .345 | .258 |
| 32 | .967 | .950 | .927 | .893 | .847 | .785 | .700 | .615 | .513 | .411 | .316 | .233 | .168 | .117 |
| 33 | .917 | .880 | .829 | .762 | .679 | .583 | .480 | .379 | .288 | .211 | .150 | .104 | .071 | .048 |
| 34 | .809 | .737 | .650 | .551 | .447 | .349 | .261 | .189 | .134 | .093 | .063 | .043 | .029 | .019 |
| 35 | .619 | .518 | .415 | .319 | .236 | .170 | .119 | .082 | .056 | .038 | .025 | .017 | .011 | .007 |
| 36 | .383 | .291 | .213 | .152 | .106 | .073 | .049 | .033 | .022 | .015 | .010 | .006 | .004 | .003 |
| 37 | .192 | .136 | .094 | .064 | .043 | .029 | .019 | .013 | .009 | .006 | .004 | .002 | .002 | .001 |
| 38 | .083 | .057 | .038 | .026 | .017 | .011 | .008 | .005 | .003 | .002 | .001 | .001 | .001 | <.001 |
| 39 | .034 | .022 | .015 | .010 | .007 | .004 | .003 | .002 | .001 | .001 | .001 | <.001 | <.001 | <.001 |
| 40 | .013 | .009 | .006 | .004 | .003 | .002 | .001 | .001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Values are expressed as probabilities. Bold values represent risk cutoff of less than 0.030 or 3%.
DISCUSSION
Optimal timing of delivery is a critical determinant of perinatal outcome. When faced with a pregnancy complication and possibility of a premature birth, knowledge of the lung maturity status of the fetus may assist the obstetric care provider in deciding about timing of delivery. Amniocentesis and use of one or more biochemical tests to measure the surface-active properties of surfactant phospholipids secreted by the fetal lungs into the amniotic fluid remains the only reliable technique for predicting risk for neonatal RDS. Since its development in the early 1970s,7,8 the L/S ratio has become one of the best validated and most commonly used biochemical tests to predict fetal lung maturity. It remains the test of choice in many institutions throughout the United States. A cutoff of 2.0 or 2.5 is generally regarded as being predictive of pulmonary maturity without regard to gestational age. Gestational age is an important determinant of RDS2–6; as such, having a single L/S value cutoff for all gestational ages may not be reasonable. Gestational age-specific cutoffs for the prediction of RDS have previously been described for many other fetal lung maturity assays,11–16 but no such analysis has previously been done for the L/S ratio.
By modeling the odds of neonatal RDS using multivariate logistic regression based on our study population, we have confirmed that both L/S ratio and gestational age are independent predictors of RDS. The data demonstrate that lower L/S ratio cutoff values can be used at increasing gestational ages to predict the same risk of RDS. For example, a probability of RDS of 3% or less was noted at an L/S ratio cutoff of ≥3.4 at 34 weeks, ≥2.6 at 36 weeks, ≥1.6 at 38 weeks, and ≥1.2 at term. Stated differently, the L/S value cutoff conferring the same probability of RDS within the clinically important gestational age window of 32 to 37 weeks ranges from 3.4 to 1.2. Above 38 weeks, RDS is so unusual that an L/S ratio as low as 1.2 would still confer a less than 3% probability of RDS. Similarly, at a gestational age of < 34 weeks, the risk of RDS is so high that a probability of RDS as low as 3% is not yielded by our algorithm. At 31 weeks, an L/S ratio of 3.6 in our model yields a probability of RDS of ~25%, highlighting the potential risk of proceeding with delivery at lower gestational ages, even when the L/S ratio is greater than 2.0. The lowest gestational age at which we have data (26 weeks) yields a probability of RDS of 97 to 99% irrespective of the L/S ratio. Table 3 summarizes the gestational age-specific risks of RDS by L/S ratio results that may be relevant when counseling patients about the risk of RDS. These data further suggest that there may be little clinical utility to amniocentesis and measurement of amniotic fluid L/S ratio prior to 31 weeks’ gestation.
This study is limited by sample size. Even though we included consecutive patients over a 7-year period undergoing amniocentesis for fetal lung maturity at a major tertiary care center that uses L/S ratio as a primary test, the requirement that delivery occur within 72 hours of the test result limited the number of subjects and cases of RDS. If the L/S ratio was suggestive of pulmonary immaturity, delivery was often delayed and amniocentesis repeated at a later date. This naturally limits the potential to correlate RDS temporally with L/S ratios. With only eight cases of RDS in our study sample, the confidence intervals surrounding our predictions for probability of RDS in Table 3 are wide. Our decision to include only women who delivered within 72 hours of amniocentesis resulted in most of the exclusions (n = 158) and significantly impacted the number of subjects in our study, but was necessary to accurately correlate L/S ratio values with probability of RDS. The small number of RDS cases also explains why we were unable to confirm previous reported associations between risk of neonatal RDS and, among others, African-American ethnicity,17 male gender, antenatal tobacco use,18 preeclampsia,19 and diabetes mellitus.20 Interestingly, prior mathematical modeling has shown that these factors, although likely associated with neonatal RDS, have a very minor effect when compared with gestational age and fetal lung maturity tests.21 Likewise, the small number of cases precluded subgroup analysis by conditions such as preterm premature rupture of membranes and preterm labor.
Administration of antenatal corticosteroids is known to protect against the development of RDS in infants delivered prior to 34 weeks of gestation,9,10 but there are no consistent data showing benefit to routine administration of antenatal steroids in preventing RDS after 34 weeks. Moreover, the usual response to an “immature” L/S ratio test after 34 weeks is not to administer steroids, but to delay delivery and repeat the L/S ratio measurement a week later. Because the mean gestational ages of the two groups were different, the observation that more women in the RDS group received antenatal steroids than in the non-RDS group is likely a marker of earlier gestational age and not an independent predictor of neonatal RDS.
In conclusion, this study shows for the first time that gestational age-specific cutoffs for the amniotic fluid L/S ratio may be important in predicting risk of neonatal RDS. These data confirm that antenatal prediction of RDS is not a dichotomous endeavor, but represents a continuum of risk depending on gestational age, biochemical assays (such as the L/S ratio), and clinical factors. A better understanding of the risk of neonatal RDS based on these criteria will aid clinical decision making and lead to improvements in neonatal outcome.
ACKNOWLEDGMENTS
We thank Jennifer Pigali for her assistance in providing neonatal outcome data. Dr. Illuzzi receives support from NICHD as a Women’s Reproductive Health Research Scholar (NIH K 12 HD047918).
REFERENCES
- 1.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959;97(5, Part 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med. 2001;164:1154–1160. doi: 10.1164/ajrccm.164.7.2012126. [DOI] [PubMed] [Google Scholar]
- 3.Robertson PA, Sniderman SH, Laros RK, Jr, et al. Neonatal morbidity according to gestational age and birth weight from five tertiary care centers in the United States, 1983 through 1986. Am J Obstet Gynecol. 1992;166(6 Pt 1):1629–1641. doi: 10.1016/0002-9378(92)91551-k. [DOI] [PubMed] [Google Scholar]
- 4.ACOG Educational Bulletin. Assessment of fetal lung maturity. Number 230, November 1996. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1997;56:191–198. [PubMed] [Google Scholar]
- 5.Copper RL, Goldenberg RL, Creasy RK, et al. A multicenter study of preterm birth weight and gestational age-specific neonatal mortality. Am J Obstet Gynecol. 1993;168(1 Pt 1):78–84. doi: 10.1016/s0002-9378(12)90889-3. [DOI] [PubMed] [Google Scholar]
- 6.Usher RH, Allen AC, McLean FH. Risk of respiratory distress syndrome related to gestational age, route of delivery, and maternal diabetes. Am J Obstet Gynecol. 1971;111:826–832. doi: 10.1016/0002-9378(71)90495-9. [DOI] [PubMed] [Google Scholar]
- 7.Gluck L, Kulovich MV, Borer RC, Jr, Brenner PH, Anderson GG, Spellacy WN. Diagnosis of the respiratory distress syndrome by amniocentesis. Am J Obstet Gynecol. 1971;109:440–445. doi: 10.1016/0002-9378(71)90342-5. [DOI] [PubMed] [Google Scholar]
- 8.Gluck L, Kulovich MV, Borer RC, Jr, Keidel WN. The interpretation and significance of the lecithin-sphingomyelin ratio in amniotic fluid. Am J Obstet Gynecol. 1974;120:142–155. doi: 10.1016/0002-9378(74)90194-x. [DOI] [PubMed] [Google Scholar]
- 9.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1–24. [PubMed] [Google Scholar]
- 10.ACOG Technical Bulletin Number 210. Washington DC: ACOG; 1998. Antenatal corticosteroid therapy for fetal maturation. [Google Scholar]
- 11.McElrath TF, Colon I, Hecht J, Tanasjavic MJ, Norwitz ER. Neonatal respiratory distress syndrome as a function of gestational age and an assay for surface-to-albumin ratio. Obstet Gynecol. 2004;103:463–468. doi: 10.1097/01.AOG.0000113622.82144.73. [DOI] [PubMed] [Google Scholar]
- 12.Tanasijevic MJ, Wybenga DR, Richardson D, Greene MF, Lopez R, Winkelman JW. A predictive model for fetal lung maturity employing gestational age and test results. Am J Clin Pathol. 1994;102:788–793. doi: 10.1093/ajcp/102.6.788. [DOI] [PubMed] [Google Scholar]
- 13.Parvin CA, Kaplan LA, Chapman JF, McManamon TG, Gronowski AM. Predicting respiratory distress syndrome using gestational age and fetal lung maturity by fluorescent polarization. Am J Obstet Gynecol. 2005;192:199–207. doi: 10.1016/j.ajog.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Pinette MG, Blackstone J, Wax JR, Cartin A. Fetal lung maturity indices—a plea for gestational age-specific interpretation: a case report and discussion. Am J Obstet Gynecol. 2002;187:1721–1722. doi: 10.1067/mob.2002.128089. [DOI] [PubMed] [Google Scholar]
- 15.Karcher R, Sykes E, Batton D, et al. Gestational age-specific predicted risk of neonatal respiratory distress syndrome using lamellar body count and surfactant-to-albumin ratio in amniotic fluid. Am J Obstet Gynecol. 2005;193:1680–1684. doi: 10.1016/j.ajog.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 16.Ventolini G, Neiger R, Hood DL, Belcastro MR. Changes in the threshold of fetal lung maturity testing and neonatal outcome of infants delivered electively before 39 weeks gestation: implications and cost-effectiveness. J Perinatol. 2006;26:264–267. doi: 10.1038/sj.jp.7211501. [DOI] [PubMed] [Google Scholar]
- 17.Berman S, Tanasijevic MJ, Alvarez JG, Ludmir J, Lieberman E, Richardson DK. Racial differences in the predictive value of the TDx fetal lung maturity assay. Am J Obstet Gynecol. 1996;175:73–77. doi: 10.1016/s0002-9378(96)70253-3. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman E, Torday J, Barbieri R, Cohen A, Van Vunakis H, Weiss ST. Association of intrauterine cigarette smoke exposure with indices of fetal lung maturation. Obstet Gynecol. 1992;79:564–570. [PubMed] [Google Scholar]
- 19.Winn HN, Klosterman A, Amon E, Shumway JB, Artal R. Does preeclampsia influence fetal lung maturity? J Perinat Med. 2000;28:210–213. doi: 10.1515/JPM.2000.028. [DOI] [PubMed] [Google Scholar]
- 20.Piper JM, Xenakis EM, Langer O. Delayed appearance of pulmonary maturation markers is associated with poor glucose control in diabetic pregnancies. J Matern Fetal Med. 1998;7:148–153. doi: 10.1002/(SICI)1520-6661(199805/06)7:3<148::AID-MFM9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Melanson SE, Berg A, Jarolim P, Tanasijevic MJ, McElrath TF. Validation of a formula that calculates the estimated risk of respiratory distress syndrome. Obstet Gynecol. 2006;108:1471–1476. doi: 10.1097/01.AOG.0000245785.70216.8a. [DOI] [PubMed] [Google Scholar]