Abstract
The aim of gene therapy for cystic fibrosis (CF) lung disease is to efficiently and safely express the CF transmembrane conductance regulator (CFTR) in the appropriate pulmonary cell types. Although CF patients experience multi-organ disease, the chronic bacterial lung infections and associated inflammation are the primary cause of shortened life expectancy. Gene transfer-based therapeutic approaches are feasible, in part, because the airway epithelium is directly accessible by aerosol delivery or instillation. Improvements in standard delivery vectors and the development of novel vectors, as well as emerging technologies and new animal models, are propelling exciting new research forward. Here, we review recent developments that are advancing this field of investigation.
INTRODUCTION
Since the discovery of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) gene in 1989, CF has been in the sights of scientists hoping to prevent or delay the onset and progression of lung disease through the use of gene transfer. Although loss of CFTR function adversely affects multiple cells and tissues, progressive lung disease accounts for the majority of the morbidity and mortality. For this reason, most effort in the field has focused on gene transfer to the airways. CFTR is expressed in multiple epithelial cell types in the surface and submucosal glands of the conducting airways where its mRNA is expressed in low abundance. The gene product is an apical membrane anion channel that is regulated by nucleotides and phosphorylation (1–3). Loss of CFTR function likely alters the volume and composition of airway secretions, but key details of the molecular pathogenesis of CF lung disease remain the subject of intense study.
Complementation of this autosomal recessive disease by the delivery of a CFTR cDNA to the airway epithelium with a viral or non-viral vector holds appeal, as the envisioned target cells are accessible via direct instillation or aerosol delivery approaches. Furthermore, early studies indicated that complementation of as few as 6–10% of CF epithelia generated wild-type levels of chloride transport in vitro (4). However, since the completion of the first human gene therapy trial in 1993, the achievement of this goal has proved challenging.
There is controversy regarding which cells to target for CF gene therapy. Arguments can be made in support of correcting cells of the surface epithelium, the submucosal glands, or both (5–8). A heterogeneous population of cell types express CFTR in the airways, including ciliated cells within the surface epithelium and a subpopulation of cells in submucosal gland ducts and acini. There appear to be several epithelial cell types in the lung that provide progenitor functions, providing the possibility of long-term correction if such cells can be targeted with integrating vectors (9). These cells may represent a pluripotent population or serve as progenitors for a specific lineage. Experiments from several species and model systems identify potential progenitor populations, including: basal cells (10,11) and non-ciliated columnar cells of the airways (10,12,13), submucosal gland epithelia (14–16), Clara cells (17,18) and alveolar type II cells in the distal lung (19,20). Studies using integrating vectors [Moloney murine leukemia virus (MLV) and lentivirus based] suggest that if cells with progenitor capacity are targeted in vitro and in vivo, long-term expression can be attained (21–25).
RECENT VECTOR DEVELOPMENTS
Enveloped viral vectors
Current lentiviral vector technology has made considerable progress toward the aims of efficiently, safely, and persistently expressing CFTR in the appropriate pulmonary cell types. Studies are beginning to examine the consequences of repeated administration of lentiviral-based vectors in the airways. Sinn et al. (26) repeatedly administered (seven doses, one dose/week) a baculovirus envelope (GP64)-pseudotyped feline immunodeficiency virus (FIV)-based lentiviral vector to the nasal epithelia of mice and observed dose-dependent increases in reporter gene expression that persisted for the 80-week duration of the experiment (Fig. 1). The observed innate or adaptive immune responses to the vector or vector-encoded transgenes were minimal and failed to curtail reporter or therapeutic gene expression. In contrast, Limberis et al. (27) reported that gene transfer with a VSV-G-pseudotyped HIV vector resulted in activation of transgene-specific T cells in mice. Transduction by VSV-G-pseudotyped HIV vectors can be further improved by formulations including magnetofectins (28), polyethylenimine (29) or lysophosphatidylcholine (30,31). Such methods may prove vital for achieving transduction efficiencies sufficient to correct the chloride transport defect in vivo. Careful preclinical studies in large animal models will be needed to further assess the safety and efficacy of the different lentiviral vector platforms under investigation for pulmonary gene transfer.
Figure 1.
Repeat administration of lentiviral vector results in persistent transgene expression in the nasal airways. Seven doses of GP64-pseudotyped FIV expressing luciferase were delivered at 1-week intervals for 7 consecutive weeks to mice via nasal instillation (arrows). At the indicated time points, light release was captured using bioluminescent imaging and data quantified with Living Image software. Stable luciferase expression was documented for 80 weeks. Data are expressed as mean ± standard error. n = 5.
Fetal and neonatal re-administration of a GP64-pseudotyped HIV vector was also investigated in the mouse lung (32). Buckley et al. (32) compared a single fetal intra-amniotic administration, one fetal and two neonatal administrations and two neonatal administrations. The levels of macrophage transduction increased with neonatal re-administration. The authors speculated that following the initial dose of lentiviral vector, macrophages are recruited to the pulmonary lumen and are subsequently transduced by the second and third doses. The authors further concluded that intra-amniotic administration of GP64-pseudotyped HIV was the most efficient mode of delivery for achieving airway epithelial cell transduction in the mouse model.
Mitomo et al. (33) described a simian immunodeficiency virus (SIV)-based lentiviral vector pseudotyped with the Sendai virus envelope proteins, hemaglutinin-neuraminidase (HN) and fusion (F) protein. F/HN-pseudotyped SIV vector transduced nasal epithelial cells, resulting in sustained transgene expression for the duration of the experiment (8–12 months) in vivo. Similar to studies with GP64-pseudotyped FIV (26), re-administration was feasible with F/HN-pseudotyped SIV, where transgene expression remained stable after three vector doses. In addition, F/HN-pseudotyped SIV conferred functional CFTR expression in vitro as determined by iodide efflux assay.
Paramyxovirus family members with known airway tropism are currently being explored as potential CFTR delivery vehicles for the treatment of CF lung disease using reverse genetics systems. Kwilas et al. (34) recently demonstrated that a respiratory syncytial virus (RSV)-based vector could deliver CFTR and correct the chloride transport defect in primary cultures of human CF airway epithelia. In addition, a human parainfluenza virus (PIV)-based vector mediated detectable but transient expression of GFP and α-fetoprotein in rhesus macaques (35). Both the RSV- and PIV-based vectors are replication competent; however, these studies may lead to replication-attenuated vectors that are further engineered to reduce the expression of cytotoxic and/or immunogenic proteins. If such engineering is feasible, it could improve the duration of gene expression, address the obstacle of pre-existing immunity, allow for repeat administration and make these vectors suitable for clinical studies.
Encapsidated viral vectors
Helper-dependent adenoviral (HD-Ad) vectors do not express viral-coding sequences and elicit reduced cell-mediated immune responses, compared with earlier generations of Ad vectors. However, HD-Ad capsid proteins remain targets for neutralizing antibodies and may trigger cytokine responses from innate immune effector cells. Recently, Cao et al. (36) demonstrated that transient immunosuppression significantly enhanced the efficiency of transgene expression and facilitated re-administration of HD-Ad vectors to mouse lungs. In addition to immunosuppression, serotype switching is a proposed technique to allow for redosing of CFTR expressing HD-Ad vectors in vivo (37). Granio et al. (38) delivered an Ad vector expressing GFP-tagged CFTR to primary cultures of human CF airway epithelia. They observed that swapping Ad5 fiber with serotype 35 fiber conferred more effective apical transduction and correction of the Cl− transport defect. These data suggest that Ad vectors such as Ad5 that use the coxackie and adenovirus receptor (CAR) are less effective at transducing the apical surface of airway epithelial cells than CAR-independent vector serotypes such as Ad35. Taken together, these studies outline strategies for using HD-Ad, immunosuppression, serotype switching and optimal fiber selection to improve the safety and long-term efficacy of adenovirus for gene transfer to the airways.
Recombinant adeno-associated virus (AAV) has been used for pulmonary gene transfer in several preclinical and clinical trials. Flotte et al. (39) demonstrated that AAV1 offered advantages over AAV5 in the chimpanzee airways, in terms of both gene transfer efficiency and reduced immunogenicity. Importantly, this observation was validated by studies in well-differentiated human airway epithelia, suggesting that the dual reporter virus co-infection approach can help predict efficacy of AAV vectors in vivo. Progress has also been made in engineering minimal CFTR expression cassettes that can be accommodated by the AAV vector (40).
Additional strategies to improve the efficiency of AAV transduction to airway epithelia include using different capsid serotypes or capsid mutants with a greater affinity for airway epithelial cells (41). As discussed in what follows, other novel AAV capsid variants have resulted from directed evolution and sequence shuffling (42–44). Although standard triple transfection methodology remains an option, new developments in baculovirus-based methods are better suited to meet AAV production requirements (45–47). In conclusion, improvements in AAV engineering, capsid serotype design and production methods have made AAV an attractive vector choice for delivering CFTR to the airways. Studies of efficacy and vector re-administration in large animal models will help guide vector development.
Non-viral vectors
Considerable progress has been made toward developing non-viral vectors for gene transfer to the lung. Typically, non-viral vectors fall into two categories: (i) non-integrating, such as plasmids (48), nanoparticles (49) and mini-circles (50), or (ii) integrating, such as transposons (51) and phage phiC31 (52,53). Both integrating and non-integrating non-viral vectors face many of the same delivery and transduction obstacles in vivo. Optimizing the delivery efficiency of DNA-based vectors to the in vivo airways remains a focus of the field (54,55). Doxorubicin (56), carboxymethylcellulose (57) and chitosan (58) improve plasmid-based gene transfer and expression in the airways. As an alternative, some groups are investigating hybrid vector systems combining features of adenovirus (59) or lentivirus with transposon-based vectors to improve delivery (60).
RECENT DEVELOPMENT OF CLINICAL STUDIES FOR CF GENE THERAPY
Since 1993, approximately 25 phase I/II trials using either viral or non-viral vectors for CF have been conducted (61). A currently ongoing clinical trial in the field was initiated by the UK CF Gene Therapy Consortium, funded by the Cystic Fibrosis Trust, using an aerosolized non-viral gene transfer agent (62). CF patients are receiving a single dose of a plasmid carrying the CFTR cDNA that is complexed to the cationic lipid GL67. The plasmid, termed pGM169, is devoid of putative pro-inflammatory sequences (CpG islands), and gene expression is regulated by a hybrid elongation factor-1a promoter. When complexed to the cationic lipid GL67A, pGM169 led to >4-week expression in mouse models upon single dosing (48). The initial single-dose clinical trial will assess safety and duration of expression in patients and will guide a planned (approximately 100 patients) multi-dose placebo-controlled trial. The planned trial, due to start in autumn 2011 (Uta Griesenbach, personal communication), will determine whether repeated non-viral CFTR gene transfer (12 doses over 12 months) improves CF lung disease (61).
NEW TECHNOLOGIES
Directed evolution of viral vectors
As vehicles for gene therapy applications, all viral vectors have potential weaknesses, such as immunogenicity, tropism, transient transgene expression and production to high titers. The success in exploiting viral vectors will depend on the ability to overcome these limitations. Directed evolution of viruses is a method for generating new or improved viral protein properties using selection-based approaches. AAV and retroviruses have been the subject of combinatorial engineering approaches in the past decade. AAV has been attractive due to its safety profile, low immunogenicity and ability to transduce both dividing and non-dividing cells. The AAV capsid determines infectivity and cell tropism (63) and is therefore the target of modification by directed evolution (42) or phage panning (64). The breadth of naturally occurring AAV serotypes suggests that the capsid is tolerant to changes (65). Directed evolution of the AAV capsid by PCR-based mutagenesis combined with high-throughput in vitro recombination generated a library of chimeric cap genes with components from two diverse serotypes. These serotypes, AAV2 and AAV5, use distinct receptors, heparan sulfate and sialic acid, respectively (42). Further selection of this library for improved transduction of human airway cells in culture identified a novel AAV variant, AAV2.5T, a chimera between AAV2 (aa1–128) and AAV5 (aa129–725) with a single-point mutation (A581T) that exhibited enhanced binding to the apical surface of airway epithelia and improved gene transfer (42). Furthermore, AAV2.5T could efficiently express the CFTR cDNA in human airway cells in culture and correct the Cl− transport defect in human CF epithelia (42).
Gamma retrovirus vectors are also efficient gene delivery tools but insertional mutagenesis and potential oncogenesis due to preferential integration at transcriptional start sites (TSS) are limitations for their clinical use (66–69). Lim et al. (70) recently showed that the random insertion of engineered zinc finger domains throughout the MLV Gag-Pol region and selection of viable variants resulted in a shifting of the integration preferences of these vectors. Furthermore, these modified integration patterns did not favor TSS. This approach could be extended to lentiviruses and may serve as a powerful method to improve the safety profile of retroviruses as gene transfer vectors for clinical use.
Gene repair and gene addition
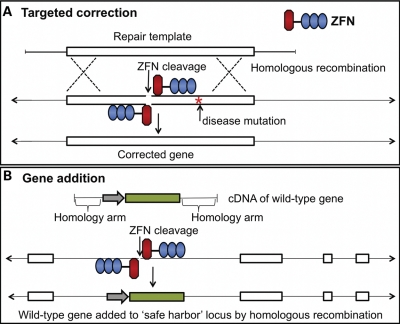
A technique known as ‘genome editing’ enables efficient and precise modification of a target sequence in a genome by introduction of a double-strand break (DSB) followed by modification of the locus during subsequent DSB repair by homologous recombination. The DSB is induced by a zinc finger nuclease (ZFN) (71–74), a specifically engineered endonuclease designed to cleave a chosen target in the genome. The ZFN consists of two components: the zinc finger protein (ZFP) and the non-specific cleavage domain of Fok1 endonuclease. The ZFP binds to the target sequence and contains a tandem array of Cys2-His2 fingers (75) each recognizing 3 bp of DNA. The arrays generally contain three or four fingers that bind a 9 or 12 bp target, respectively. The Fok1 domains must dimerize to cleave the DNA (76), consequently the specificity of the recognition site is doubled from 9 to 18 bp for a three-finger ZFN.
Homology-based genome editing can be exploited for correction of mutated genes responsible for monogenic disorders (Fig. 2). ZFN-based genome editing requires delivery of a donor DNA repair template along with the target specific ZFN pair. Methods for generating ZFN pairs targeting specific genomic loci are becoming widely available and include modular design approaches (77–79) and the selection-based oligomerized pool engineering (OPEN) strategy (80). ZFNs designed using OPEN technology have been shown to bind the genomic DNA-encoding CFTR (78) and to create DSBs near the ▵F508 mutation in exon 10. Provision of a wild-type donor DNA template with a non-integrating vector, such as integrase-deficient lentiviral vector (81), can facilitate repair of this mutation by homologous recombination.
Figure 2.
Schematic of targeted gene correction and gene addition strategies. (A) Expressed ZFN pairs bind to and cleave the target genomic locus near the disease-causing mutation. ZFNs are composed of chimeric zinc fingers (blue) and Fok1 endonuclease domains (red). Introduction of a DSB promotes homologous recombination, using the repair template donor. (B) For targeted gene addition, the ZFN pair cleaves a predetermined ‘safe harbor' locus. The expression cassette with a therapeutic gene (green) is flanked by homology arms to the safe harbor locus.
Other repair strategies using homing endonucleases (82–84) or transcription activator-like effector nucleases (85) provide alternative mechanisms for creating DSBs in genomic DNA and allowing for gene repair by co-delivery of a homology repair template. A potential advantage of each of these gene repair approaches is that correction of CFTR in progenitor cell types could preserve the native regulatory elements and allow for correction in subsequent daughter cells. Reagent development and delivery to airway epithelia will be important for this field to advance.
An alternative to the gene repair approach is termed ‘targeted gene addition’ (Fig. 2). Here, ZFNs may be used to create DSBs at potential ‘safe harbor’ loci such as AAVS1 (86), CCR5 (87) or the mouse Rosa26 locus (88). In this approach, the entire therapeutic transgene with flanking homology arms would be inserted into the safe harbor loci by homologous recombination. Modification of such a locus is reasoned much less likely to perturb expression of neighboring genes or disrupt the function of other genetic elements (89).
Applications of RNA interference to treat CF
The recent explosion of knowledge in the field of small interfering RNAs has led to applications of direct relevance to CF. First, RNAi has been used as a tool to identify gene products that contribute to steps in wild-type and mutant CFTR biogenesis, including ER and Golgi trafficking, residence time in the cell membrane and its removal by proteosomal degradation (90–92). This has raised the possibility that RNAi-based strategies might be developed to increase the expression of ▵F508 CFTR, to rescue ▵F508 CFTR from proteosomal degradation or enhance its residence time in the cell membrane. Any of these approaches might provide sufficient residual CFTR function to be therapeutically relevant. Similarly, targeting other cellular pathways, such as those involved in inflammation, might offer a means to ameliorate disease symptoms and progression. A significant hurdle for translational studies in this area is identifying the methods to efficiently deliver RNAi to well-differentiated airway epithelia. Another area of investigation relevant to the field is the identification of the microRNA repertoire in airway epithelia and other CFTR expressing cells, as well as their respective target gene products. Knowledge in this area may identify new targets for therapeutic manipulation.
Lung tissue engineering
Lung transplantation is currently the only definitive treatment for end-stage CF lung disease. The supply of donor lungs is limited and transplantation achieves only a 10–20% survival at 10 years (93). Recently, two groups independently used similar tissue-engineering strategies to develop an autologous bioartificial lung that may begin to help overcome the limited availability of donor tissues (94,95). The bioartificial lungs were created by first generating a whole-lung scaffold by perfusion and decellularization of the adult rat lung, followed by reseeding of the endothelial and epithelial surfaces of the scaffold with new cells. Evidence for gas exchange within the resulting grafts was demonstrated. With the further development of this technology, one could envision the ex vivo correction of patient-derived cells, followed by lung tissue engineering and transplantation. Although these initial results are very exciting, several steps need to be further optimized before long-term tissue-engineered lung function can be translated to the clinic (96).
NEW ANIMAL MODELS OF CF DISEASE
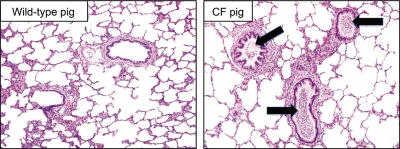
A significant bottleneck in the development of new CF therapeutics has been the lack of animal models that recapitulate key features of lung and other organ disease pathogenesis. The mouse models with CFTR null alleles and specific disease mutations available since the early 1990s have contributed greatly to disease understanding but fail to develop spontaneous lung disease similar to humans with CF. Recently, two groups used somatic cell targeting of the CFTR gene with AAV vectors, followed by nuclear transfer and cloning to develop novel models in pigs (97,98) and ferrets (99,100). These new animal models recapitulate key features of CF disease (98,100). At birth, the lungs of CFTR null pigs are free of inflammation but manifest a bacterial host defense defect without the secondary consequences of infection (98,101). CF pigs spontaneously develop a lung disease phenotype mirroring key features of human CF lung disease in the first months of life, including infection with bacteria, airway remodeling and mucus hypersecretion (Fig. 3). CFTR null ferrets also develop multi-organ system disease, and neonatal animals manifest a pulmonary host defense defect in the airways associated with colonization by bacteria (100). There is also early evidence that adult CF ferrets develop a lung disease phenotype with similarities to human CF, including bacterial colonization (John Engelhardt, personal communication). These phenotypic features make these new models very attractive for gene therapy studies. They offer the unique opportunity to test gene therapy interventions prior to the onset of lung disease and monitor the outcomes for prevention-based treatment strategies.
Figure 3.
Pigs with CFTR mutations develop pulmonary disease with similarity to humans with CF. In this example from a pig of 5.5 months of age, key pathogenic features observed include mucus accumulation in the airways, airway obstruction with purulent material containing neutrophils and bacteria (right panel, black arrows) and airway remodeling. The bacterial cultures from this animal were positive for Bordetella bronchiseptica. The wild-type pig is an age-matched control. The stain used was hematoxylin and eosin. Both images are of ×100 magnification.
CONCLUSIONS
This is an unprecedented time in the development of new therapies for CF. The near-universal availability of newborn screening for CF in developed nations has made early diagnosis commonplace, allowing the potential for treatment of healthy lungs before the onset of chronic lung disease. However, this opportunity comes at a time where there is a dearth of sensitive and specific markers of early disease that can be used to assess lung disease onset and monitor responses to therapy. Additional work is needed to develop new specific and sensitive measures of the early stages of lung disease suitable for monitoring the response to therapies for use in infants and young children. Parallel developments in improved gene transfer tools should further aid the field and lead to new clinical trials.
FUNDING
This work was supported by NIH grants: R01 HL-075363 (P.B.M.), PO1 HL-51670 (P.B.M.), R21 HL-91808 (P.B.M.), the Cystic Fibrosis Foundation (P.L.S.) and the Roy J. Carver Charitable Trust (P.B.M.). We also acknowledge the support of the In Vitro Models and Cell Culture Core and Cell Morphology Cores, partially supported by the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759) and the Cystic Fibrosis Foundation.
ACKNOWLEDGEMENTS
We thank John Engelhardt and Uta Griesenbach for their helpful discussions, as well as David Meyerholz for providing the images presented in Figure 3.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Sheppard D.N., Welsh M.J. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 2.Devidas S., Guggino W.B. CFTR: domains, structure, and function. J. Bioenerg. Biomembr. 1997;29:443–451. doi: 10.1023/a:1022430906284. doi:10.1023/A:1022430906284. [DOI] [PubMed] [Google Scholar]
- 3.Riordan J.R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. doi:10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 4.Johnson L.G., Olsen J.C., Sarkadi B., Moore K.L., Swanstrom R., Boucher R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. doi:10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt J.F., Yankaskas J.R., Ernst S.A., Yang Y., Marino C.R., Boucher R.C., Cohn J.A., Wilson J.M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat. Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. doi:10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L., Dey C.R., Wert S.E., DuVall M.D., Frizzell R.A., Whitsett J.A. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. doi:10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 7.Joo N.S., Cho H.J., Khansaheb M., Wine J.J. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J. Clin. Invest. 2010;120:3161–3166. doi: 10.1172/JCI43466. doi:10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee R.J., Foskett J.K. Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 2010;298:L210–L231. doi: 10.1152/ajplung.00342.2009. doi:10.1152/ajplung.00342.2009. [DOI] [PubMed] [Google Scholar]
- 9.Liu X., Luo M., Guo C., Yan Z., Wang Y., Lei-Butters D.C., Engelhardt J.F. Analysis of adeno-associated virus progenitor cell transduction in mouse lung. Mol. Ther. 2009;17:285–293. doi: 10.1038/mt.2008.248. doi:10.1038/mt.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. doi:10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. doi:10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randell S.H. Progenitor-progeny relationships in airway epithelium. Chest. 1992;101:11S–16S. doi: 10.1378/chest.101.3_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 13.Ford J.R., Terzaghi-Howe M. Basal cells are the progenitors of primary tracheal epithelial cell cultures. Exp. Cell Res. 1992;198:69–77. doi: 10.1016/0014-4827(92)90150-7. doi:10.1016/0014-4827(92)90150-7. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt J.F., Schlossberg H., Yankaskas J.R., Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- 15.Borthwick D.W., Shahbazian M., Krantz Q.T., Dorin J.R., Randell S.H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Engelhardt J.F. The glandular stem/progenitor cell niche in airway development and repair. Proc. Am. Thorac. Soc. 2008;5:682–688. doi: 10.1513/pats.200801-003AW. doi:10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans M.J., Johnson L.V., Stephens R.J., Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab. Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- 18.Hong K.U., Reynolds S.D., Giangreco A., Hurley C.M., Stripp B.R. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 19.Evans M.J., Cabral L.J., Stephens R.J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp. Mol. Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. doi:10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 20.Adamson I.Y., Bowden D.H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab. Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- 21.Wang G., Slepushkin V., Zabner J., Keshavjee S., Johnston J.C., Sauter S.L., Jolly D.J., Dubensky T.W., Jr, Davidson B.L., McCray P.B., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Invest. 1999;104:R55–R62. doi: 10.1172/JCI8390. doi:10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G., Sinn P.L., McCray P.B., Jr Development of retroviral vectors for gene transfer to airway epithelia. Curr. Opin. Mol. Ther. 2000;2:497–506. [PubMed] [Google Scholar]
- 23.Wang G., Slepushkin V.A., Bodner M., Zabner J., van Es H.H., Thomas P., Jolly D.J., Davidson B.L., McCray P.B., Jr Keratinocyte growth factor induces epithelial proliferation facilitates retroviral-mediated gene transfer to distal lung epithelia in vivo. J. Gene Med. 1999;1:22–30. doi: 10.1002/(sici)1521-2254(199901/02)1:1<22::aid-jgm1>3.3.co;2-o. doi:10.1002/(SICI)1521-2254(199901/02)1:1<22::AID-JGM1>3.3.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Wang G., Sinn P.L., Zabner J., McCray P.B., Jr Gene transfer to airway epithelia using feline immunodeficiency virus-based lentivirus vectors. Meth. Enzymol. 2002;346:500–514. doi: 10.1016/s0076-6879(02)46073-9. doi:10.1016/S0076-6879(02)46073-9. [DOI] [PubMed] [Google Scholar]
- 25.Sinn P.L., Burnight E.R., Hickey M.A., Blissard G.W., McCray P.B., Jr Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J. Virol. 2005;79:12818–12827. doi: 10.1128/JVI.79.20.12818-12827.2005. doi:10.1128/JVI.79.20.12818-12827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinn P.L., Arias A.C., Brogden K.A., McCray P.B., Jr Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J. Virol. 2008;82:10684–10692. doi: 10.1128/JVI.00227-08. doi:10.1128/JVI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limberis M.P., Bell C.L., Heath J., Wilson J.M. Activation of transgene-specific T cells following lentivirus-mediated gene delivery to mouse lung. Mol. Ther. 2010;18:143–150. doi: 10.1038/mt.2009.190. doi:10.1038/mt.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando C., Castellani S., Mykhaylyk O., Copreni E., Zelphati O., Plank C., Conese M. Magnetically guided lentiviral-mediated transduction of airway epithelial cells. J. Gene Med. 2010;12:747–754. doi: 10.1002/jgm.1494. doi:10.1002/jgm.1494. [DOI] [PubMed] [Google Scholar]
- 29.Castellani S., Di Gioia S., Trotta T., Maffione A.B., Conese M. Impact of lentiviral vector-mediated transduction on the tightness of a polarized model of airway epithelium and effect of cationic polymer polyethylenimine. J. Biomed. Biotechnol. 2010;2010:103976. doi: 10.1155/2010/103976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cmielewski P., Anson D.S., Parsons D.W. Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length. Respir. Res. 2010;11:84. doi: 10.1186/1465-9921-11-84. doi:10.1186/1465-9921-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocker A.G., Kremer K.L., Koldej R., Miller D.S., Anson D.S., Parsons D.W. Single-dose lentiviral gene transfer for lifetime airway gene expression. J. Gene Med. 2009;11:861–867. doi: 10.1002/jgm.1368. doi:10.1002/jgm.1368. [DOI] [PubMed] [Google Scholar]
- 32.Buckley S.M., Howe S.J., Sheard V., Ward N.J., Coutelle C., Thrasher A.J., Waddington S.N., McKay T.R. Lentiviral transduction of the murine lung provides efficient pseudotype and developmental stage-dependent cell-specific transgene expression. Gene Ther. 2008;15:1167–1175. doi: 10.1038/gt.2008.74. doi:10.1038/gt.2008.74. [DOI] [PubMed] [Google Scholar]
- 33.Mitomo K., Griesenbach U., Inoue M., Somerton L., Meng C., Akiba E., Tabata T., Ueda Y., Frankel G.M., Farley R., et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol. Ther. 2010;18:1173–1182. doi: 10.1038/mt.2010.13. doi:10.1038/mt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwilas A.R., Yednak M.A., Zhang L., Liesman R., Collins P.L., Pickles R.J., Peeples M.E. Respiratory syncytial virus engineered to express the cystic fibrosis transmembrane conductance regulator corrects the bioelectric phenotype of human cystic fibrosis airway epithelium in vitro. J. Virol. 2010;84:7770–7781. doi: 10.1128/JVI.00346-10. doi:10.1128/JVI.00346-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L., Limberis M.P., Thompson C., Antunes M.B., Luongo C., Wilson J.M., Collins P.L., Pickles R.J. Alpha-fetoprotein gene delivery to the nasal epithelium of nonhuman primates by human parainfluenza viral vectors. Hum. Gene Ther. 2010;21:1657–1664. doi: 10.1089/hum.2010.065. doi:10.1089/hum.2010.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao H., Yang T., Li X.F., Wu J., Duan C., Coates A.L., Hu J. Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression. Gene Ther. 2010;18:173–181. doi: 10.1038/gt.2010.125. doi:10.1038/gt.2010.125. [DOI] [PubMed] [Google Scholar]
- 37.Bangari D.S., Mittal S.K. Current strategies and future directions for eluding adenoviral vector immunity. Curr. Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. doi:10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granio O., Ashbourne Excoffon K.J., Henning P., Melin P., Norez C., Gonzalez G., Karp P.H., Magnusson M.K., Habib N., Lindholm L., et al. Adenovirus 5-fiber 35 chimeric vector mediates efficient apical correction of the cystic fibrosis transmembrane conductance regulator defect in cystic fibrosis primary airway epithelia. Hum. Gene Ther. 2010;21:251–269. doi: 10.1089/hum.2009.056. doi:10.1089/hum.2009.056. [DOI] [PubMed] [Google Scholar]
- 39.Flotte T.R., Fischer A.C., Goetzmann J., Mueller C., Cebotaru L., Yan Z., Wang L., Wilson J.M., Guggino W.B., Engelhardt J.F. Dual reporter comparative indexing of rAAV pseudotyped vectors in chimpanzee airway. Mol. Ther. 2010;18:594–600. doi: 10.1038/mt.2009.230. doi:10.1038/mt.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostedgaard L.S., Rokhlina T., Karp P.H., Lashmit P., Afione S., Schmidt M., Zabner J., Stinski M.F., Chiorini J.A., Welsh M.J. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc. Natl Acad. Sci. USA. 2005;102:2952–2957. doi: 10.1073/pnas.0409845102. doi:10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White A.F., Mazur M., Sorscher E.J., Zinn K.R., Ponnazhagan S. Genetic modification of adeno-associated viral vector type 2 capsid enhances gene transfer efficiency in polarized human airway epithelial cells. Hum. Gene Ther. 2008;19:1407–1414. doi: 10.1089/hum.2008.117. doi:10.1089/hum.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Excoffon K.J., Koerber J.T., Dickey D.D., Murtha M., Keshavjee S., Kaspar B.K., Zabner J., Schaffer D.V. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl Acad. Sci. USA. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. doi:10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W., Zhang L., Johnson J.S., Zhijian W., Grieger J.C., Ping-Jie X., Drouin L.M., Agbandje-McKenna M., Pickles R.J., Samulski R.J. Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium. Mol. Ther. 2009;17:2067–2077. doi: 10.1038/mt.2009.155. doi:10.1038/mt.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlon M., Toelen J., Van der Perren A., Vandenberghe L.H., Reumers V., Sbragia L., Gijsbers R., Baekelandt V., Himmelreich U., Wilson J.M., et al. Efficient gene transfer into the mouse lung by fetal intratracheal injection of rAAV2/6.2. Mol. Ther. 2010;18:2130–2138. doi: 10.1038/mt.2010.153. doi:10.1038/mt.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lock M., Alvira M., Vandenberghe L.H., Samanta A., Toelen J., Debyser Z., Wilson J.M. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. doi:10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virag T., Cecchini S., Kotin R.M. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene Ther. 2009;20:807–817. doi: 10.1089/hum.2009.092. doi:10.1089/hum.2009.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith R.H., Levy J.R., Kotin R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. doi:10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyde S.C., Pringle I.A., Abdullah S., Lawton A.E., Davies L.A., Varathalingam A., Nunez-Alonso G., Green A.M., Bazzani R.P., Sumner-Jones S.G., et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat. Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. doi:10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 49.Davis P.B., Kowalczyk T.H. Preparation and analysis of PEGylated poly-L-lysine DNA nanoparticles for gene delivery. Cold Spring Harb. Protoc. 2010;2010 doi: 10.1101/pdb.prot5419. pdb prot5419. [DOI] [PubMed] [Google Scholar]
- 50.Riu E., Grimm D., Huang Z., Kay M.A. Increased maintenance and persistence of transgenes by excision of expression cassettes from plasmid sequences in vivo. Hum. Gene Ther. 2005;16:558–570. doi: 10.1089/hum.2005.16.558. doi:10.1089/hum.2005.16.558. [DOI] [PubMed] [Google Scholar]
- 51.Mikkelsen J.G., Yant S.R., Meuse L., Huang Z., Xu H., Kay M.A. Helper-independent sleeping beauty transposon–transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo. Mol. Ther. 2003;8:654–665. doi: 10.1016/s1525-0016(03)00216-8. doi:10.1016/S1525-0016(03)00216-8. [DOI] [PubMed] [Google Scholar]
- 52.Maucksch C., Aneja M.K., Hennen E., Bohla A., Hoffmann F., Elfinger M., Rosenecker J., Rudolph C. Cell type differences in activity of the streptomyces bacteriophage phiC31 integrase. Nucleic Acids Res. 2008;36:5462–5471. doi: 10.1093/nar/gkn532. doi:10.1093/nar/gkn532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olivares E.C., Hollis R.P., Calos M.P. Phage R4 integrase mediates site-specific integration in human cells. Gene. 2001;278:167–176. doi: 10.1016/s0378-1119(01)00711-9. doi:10.1016/S0378-1119(01)00711-9. [DOI] [PubMed] [Google Scholar]
- 54.McLachlan G., Baker A., Tennant P., Gordon C., Vrettou C., Renwick L., Blundell R., Cheng S.H., Scheule R.K., Davies L., et al. Optimizing aerosol gene delivery and expression in the ovine lung. Mol. Ther. 2007;15:348–354. doi: 10.1038/sj.mt.6300058. doi:10.1038/sj.mt.6300058. [DOI] [PubMed] [Google Scholar]
- 55.Alton E.W. Use of nonviral vectors for cystic fibrosis gene therapy. Proc. Am. Thorac. Soc. 2004;1:296–301. doi: 10.1513/pats.200404-031MS. doi:10.1513/pats.200404-031MS. [DOI] [PubMed] [Google Scholar]
- 56.Griesenbach U., Meng C., Farley R., Gardner A., Brake M.A., Frankel G.M., Gruenert D.C., Cheng S.H., Scheule R.K., Alton E.W. The role of doxorubicin in non-viral gene transfer in the lung. Biomaterials. 2009;30:1971–1977. doi: 10.1016/j.biomaterials.2008.12.037. doi:10.1016/j.biomaterials.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 57.Griesenbach U., Meng C., Farley R., Wasowicz M.Y., Munkonge F.M., Chan M., Stoneham C., Sumner-Jones S.G., Pringle I.A., Gill D.R., et al. The use of carboxymethylcellulose gel to increase non-viral gene transfer in mouse airways. Biomaterials. 2010;31:2665–2672. doi: 10.1016/j.biomaterials.2009.12.005. doi:10.1016/j.biomaterials.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nydert P., Dragomir A., Hjelte L. Chitosan as a carrier for non-viral gene transfer in a cystic-fibrosis cell line. Biotechnol. Appl. Biochem. 2008;51:153–157. doi: 10.1042/BA20070197. doi:10.1042/BA20070197. [DOI] [PubMed] [Google Scholar]
- 59.Hausl M.A., Zhang W., Muther N., Rauschhuber C., Franck H.G., Merricks E.P., Nichols T.C., Kay M.A., Ehrhardt A. Hyperactive sleeping beauty transposase enables persistent phenotypic correction in mice and a canine model for hemophilia B. Mol. Ther. 2010;18:1896–1906. doi: 10.1038/mt.2010.169. doi:10.1038/mt.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vink C.A., Gaspar H.B., Gabriel R., Schmidt M., McIvor R.S., Thrasher A.J., Qasim W. Sleeping beauty transposition from nonintegrating lentivirus. Mol. Ther. 2009;17:1197–1204. doi: 10.1038/mt.2009.94. doi:10.1038/mt.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griesenbach U., Alton E.W. Cystic fibrosis gene therapy: successes, failures and hopes for the future. Expert Rev. Respir. Med. 2009;3:363–371. doi: 10.1586/ers.09.25. doi:10.1586/ers.09.25. [DOI] [PubMed] [Google Scholar]
- 62.Pringle I.A., Hyde S.C., Gill D.R. Non-viral vectors in cystic fibrosis gene therapy: recent developments and future prospects. Expert Opin. Biol. Ther. 2009;9:991–1003. doi: 10.1517/14712590903055029. doi:10.1517/14712590903055029. [DOI] [PubMed] [Google Scholar]
- 63.Asokan A., Hamra J.B., Govindasamy L., Agbandje-McKenna M., Samulski R.J. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J. Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. doi:10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y.H., Chang M., Davidson B.L. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. doi:10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. doi:10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. doi:10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 67.Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.-L., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. doi:10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt M., Carbonaro D.A., Speckmann C., Wissler M., Bohnsack J., Elder M., Aronow B.J., Nolta J.A., Kohn D.B., von Kalle C. Clonality analysis after retroviral-mediated gene transfer to CD34+ cells from the cord blood of ADA-deficient SCID neonates. Nat. Med. 2003;9:463–468. doi: 10.1038/nm844. doi:10.1038/nm844. [DOI] [PubMed] [Google Scholar]
- 69.Wu X., Li Y., Crise B., Burgess S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. doi:10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 70.Lim K.I., Klimczak R., Yu J.H., Schaffer D.V. Specific insertions of zinc finger domains into Gag-Pol yield engineered retroviral vectors with selective integration properties. Proc. Natl Acad. Sci. USA. 2010;107:12475–12480. doi: 10.1073/pnas.1001402107. doi:10.1073/pnas.1001402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim Y.G., Cha J., Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc. Natl Acad. Sci. USA. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. doi:10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bibikova M., Carroll D., Segal D.J., Trautman J.K., Smith J., Kim Y.G., Chandrasegaran S. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol. Cell Biol. 2001;21:289–297. doi: 10.1128/MCB.21.1.289-297.2001. doi:10.1128/MCB.21.1.289-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moehle E.A., Rock J.M., Lee Y.L., Jouvenot Y., DeKelver R.C., Gregory P.D., Urnov F.D., Holmes M.C. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc. Natl Acad. Sci. USA. 2007;104:3055–3060. doi: 10.1073/pnas.0611478104. doi:10.1073/pnas.0611478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doyon Y., Vo T.D., Mendel M.C., Greenberg S.G., Wang J., Xia D.F., Miller J.C., Urnov F.D., Gregory P.D., Holmes M.C. Enhancing zinc-finger-nuclease activity with improved obligate heterodimeric architectures. Nat. Methods. 2011;8:74–79. doi: 10.1038/nmeth.1539. doi:10.1038/nmeth.1539. [DOI] [PubMed] [Google Scholar]
- 75.Wolfe S.A., Nekludova L., Pabo C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. doi:10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- 76.Vanamee E.S., Santagata S., Aggarwal A.K. FokI requires two specific DNA sites for cleavage. J. Mol. Biol. 2001;309:69–78. doi: 10.1006/jmbi.2001.4635. doi:10.1006/jmbi.2001.4635. [DOI] [PubMed] [Google Scholar]
- 77.Wright D.A., Thibodeau-Beganny S., Sander J.D., Winfrey R.J., Hirsh A.S., Eichtinger M., Fu F., Porteus M.H., Dobbs D., Voytas D.F., et al. Standardized reagents and protocols for engineering zinc finger nucleases by modular assembly. Nat. Protoc. 2006;1:1637–1652. doi: 10.1038/nprot.2006.259. doi:10.1038/nprot.2006.259. [DOI] [PubMed] [Google Scholar]
- 78.Maeder M.L., Thibodeau-Beganny S., Osiak A., Wright D.A., Anthony R.M., Eichtinger M., Jiang T., Foley J.E., Winfrey R.J., Townsend J.A., et al. Rapid ‘open-source’ engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol. Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. doi:10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cathomen T., Segal D.J., Brondani V., Muller-Lerch F. Generation and functional analysis of zinc finger nucleases. Methods Mol. Biol. 2008;434:277–290. doi: 10.1007/978-1-60327-248-3_17. doi:10.1007/978-1-60327-248-3_17. [DOI] [PubMed] [Google Scholar]
- 80.Maeder M.L., Thibodeau-Beganny S., Sander J.D., Voytas D.F., Joung J.K. Oligomerized pool engineering (OPEN): an ‘open-source’ protocol for making customized zinc-finger arrays. Nat. Protoc. 2009;4:1471–1501. doi: 10.1038/nprot.2009.98. doi:10.1038/nprot.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lombardo A., Genovese P., Beausejour C.M., Colleoni S., Lee Y.L., Kim K.A., Ando D., Urnov F.D., Galli C., Gregory P.D., et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. doi:10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 82.Grizot S., Epinat J.C., Thomas S., Duclert A., Rolland S., Paques F., Duchateau P. Generation of redesigned homing endonucleases comprising DNA-binding domains derived from two different scaffolds. Nucleic Acids Res. 2010;38:2006–2018. doi: 10.1093/nar/gkp1171. doi:10.1093/nar/gkp1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grizot S., Smith J., Daboussi F., Prieto J., Redondo P., Merino N., Villate M., Thomas S., Lemaire L., Montoya G., et al. Efficient targeting of a SCID gene by an engineered single-chain homing endonuclease. Nucleic Acids Res. 2009;37:5405–5419. doi: 10.1093/nar/gkp548. doi:10.1093/nar/gkp548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marcaida M.J., Munoz I.G., Blanco F.J., Prieto J., Montoya G. Homing endonucleases: from basics to therapeutic applications. Cell Mol. Life Sci. 2010;67:727–748. doi: 10.1007/s00018-009-0188-y. doi:10.1007/s00018-009-0188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller J.C., Tan S., Qiao G., Barlow K.A., Wang J., Xia D.F., Meng X., Paschon D.E., Leung E., Hinkley S.J., et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2010;29:143–148. doi: 10.1038/nbt.1755. doi:10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 86.Hockemeyer D., Soldner F., Beard C., Gao Q., Mitalipova M., DeKelver R.C., Katibah G.E., Amora R., Boydston E.A., Zeitler B., et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. doi:10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perez E.E., Wang J., Miller J.C., Jouvenot Y., Kim K.A., Liu O., Wang N., Lee G., Bartsevich V.V., Lee Y.L., et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. doi:10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer M., de Angelis M.H., Wurst W., Kuhn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc. Natl Acad. Sci. USA. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. doi:10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papapetrou E.P., Lee G., Malani N., Setty M., Riviere I., Tirunagari L.M., Kadota K., Roth S.L., Giardina P., Viale A., et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat. Biotechnol. 2011;29:73–78. doi: 10.1038/nbt.1717. doi:10.1038/nbt.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X., Venable J., LaPointe P., Hutt D.M., Koulov A.V., Coppinger J., Gurkan C., Kellner W., Matteson J., Plutner H., et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. doi:10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 91.Hutt D.M., Herman D., Rodrigues A.P., Noel S., Pilewski J.M., Matteson J., Hoch B., Kellner W., Kelly J.W., Schmidt A., et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat. Chem. Biol. 2010;6:25–33. doi: 10.1038/nchembio.275. doi:10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okiyoneda T., Barriere H., Bagdany M., Rabeh W.M., Du K., Hohfeld J., Young J.C., Lukacs G.L. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. doi:10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orens J.B., Garrity E.R., Jr General overview of lung transplantation and review of organ allocation. Proc. Am. Thorac. Soc. 2009;6:13–19. doi: 10.1513/pats.200807-072GO. doi:10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 94.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. doi:10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., Vacanti J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16:927–933. doi: 10.1038/nm.2193. doi:10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 96.de Perrot M., Fischer S., Liu M., Imai Y., Martins S., Sakiyama S., Tabata T., Bai X.H., Waddell T.K., Davidson B.L., et al. Impact of human interleukin-10 on vector-induced inflammation and early graft function in rat lung transplantation. Am. J. Respir. Cell Mol. Biol. 2003;28:616–625. doi: 10.1165/rcmb.2002-0109OC. doi:10.1165/rcmb.2002-0109OC. [DOI] [PubMed] [Google Scholar]
- 97.Rogers C.S., Hao Y., Rokhlina T., Samuel M., Stoltz D.A., Li Y., Petroff E., Vermeer D.W., Kabel A.C., Yan Z., et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. doi:10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rogers C.S., Stoltz D.A., Meyerholz D.K., Ostedgaard L.S., Rokhlina T., Taft P.J., Rogan M.P., Pezzulo A.A., Karp P.H., Itani O.A., et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. doi:10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun X., Yan Z., Yi Y., Li Z., Lei D., Rogers C.S., Chen J., Zhang Y., Welsh M.J., Leno G.H., et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Invest. 2008;118:1578–1583. doi: 10.1172/JCI34599. doi:10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun X., Sui H., Fisher J.T., Yan Z., Liu X., Cho H.J., Joo N.S., Zhang Y., Zhou W., Yi Y., et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. doi:10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stoltz D.A., Meyerholz D.K., Pezzulo A.A., Ramachandran S., Rogan M.P., Davis G.J., Hanfland R.A., Wohlford-Lenane C., Dohrn C.L., Bartlett J.A., et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]