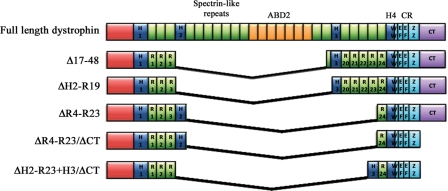
Figure 1.
Schematic representation of micro-dystrophin constructs. Full-length dystrophin consists of an actin binding domain (ABD1) at the N-terminus, four hinge domains (H1-4), 24 spectrin-like repeats (R1–R24) (within which lies a second ABD (ABD2)), a cysteine rich domain (CR) and the carboxy-terminal domain (CT). The CR itself consists of a WW domain, two EF-hand regions and a zinc finger domain (ZZ) that together form the dystroglycan binding site (122). Dystrophin also binds to α1-syntrophin and β1-syntrophin at the CT domain (123). Expression of the construct generated by deletion of exons 17–48 (Δ17–48), which was originally observed in a mildly affected Becker's muscular dystrophy patient, corrected 95% of the morphology in transgenic mdx mice and supports near normal force development (39,124). Later modification of this construct (ΔH2-R19) and one carrying deletions of R4–R23 (ΔR4–R23) have also been shown to reverse dystrophic pathology in transgenic mdx mice (41). Further truncation of dystrophin with deletion of the CT domain (ΔR4–R23/ΔCT) resulted in significant improvement in force in older dystrophic mice (45), confirming that the CT domain is not critical for dystrophin function. Recent work has now shown that replacing hinge 2 with hinge 3 in micro-dystrophin (ΔH2-R23 + H3/ΔCT) significantly improves the functional capacity of truncated dystrophins as demonstrated by prevention of muscle degeneration in mdx mice injected with rAAV6-ΔH2-R23 + H3/ΔCT (125). The size of a construct dictates which vectors can be used for delivery. The full-length cDNA only fits into gutted or high-capacity adenoviral vectors, the larger mini-dystrophins can be delivered with lentiviral vectors, while the smallest micro-dystrophins can be systemically delivered using rAAV.