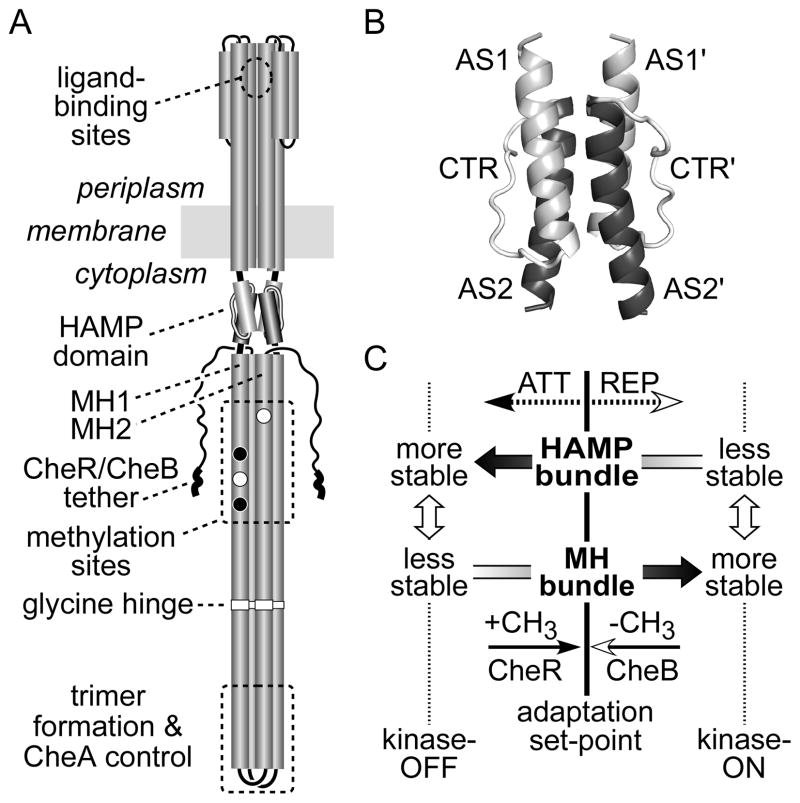
Fig. 1. Tsr and HAMP structure and our initial dynamic bundle signaling model.
(A) Important signaling features of the Tsr dimer. Cylindrical segments represent α-helical regions, drawn approximately to scale. Methylation sites shown as black circles indicate glutamine residues that must be deamidated to glutamates by CheB before accepting methyl groups; open circles represent glutamate residues that are direct substrates for the CheR methyltransferase. Thickened regions at the C-terminus of each subunit represent a pentapeptide sequence (NWETF) to which CheB and CheR bind.
(B) Probable structure of the HAMP domain in a Tsr dimer. Each HAMP subunit consists of two amphiphilic helices (AS1, AS2) joined by a nonhelical connector (CTR). The four-helix bundle is modeled on the atomic coordinates for Af1503 HAMP (Hulko et al., 2006), as described (Ames et al., 2008). The four helices pack in parallel orientation (N-termini at the top). This grayscale shading convention for the HAMP structural elements is used in all subsequent figures.
(C) Dynamic-bundle model of HAMP signaling (Zhou et al., 2009). The original model proposed that chemoeffector stimuli control signal output by modulating the packing stability of the HAMP bundle, which is oppositionally coupled to the stability of the MH1/MH1′ packing interactions. Methylation and demethylation of sites in the MH bundle shift stability in the opposing direction to reverse HAMP signaling effects during sensory adaptation.