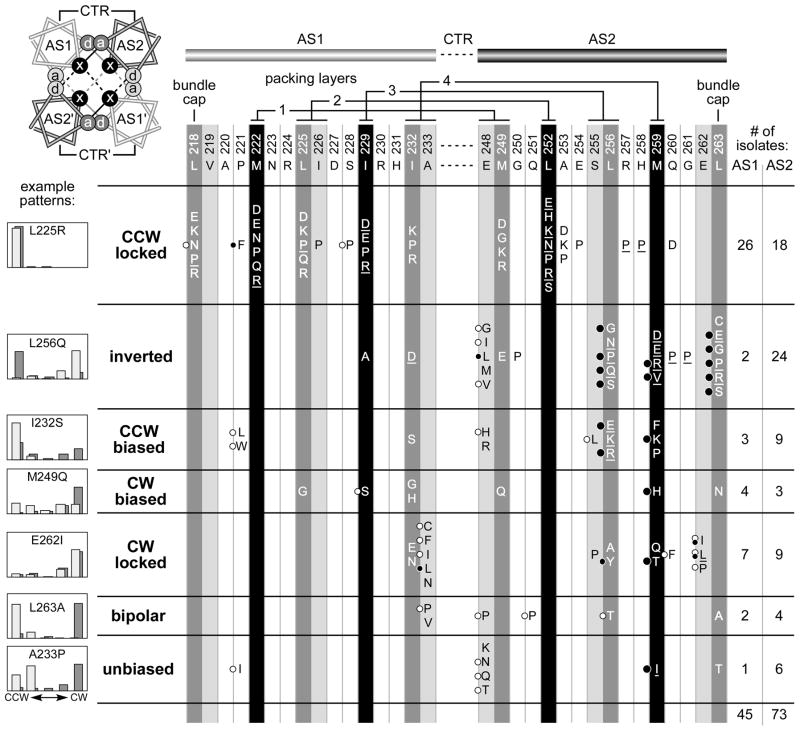
Fig. 2. Output patterns of Tsr molecules with null lesions in the AS1 and AS2 HAMP helices.
AS1 and AS2 hydrophobic residues that are most critical for signaling function are highlighted in black and dark gray (Zhou et al., 2009). The residues at light gray positions are not critical for Tsr-HAMP function, with the exception of A233 and E248 (Zhou et al., 2009), which are highly conserved HAMP features (Dunin-Horkawicz & Lupas, 2010). The helical wheel diagram at upper left views the HAMP bundle from the N-termini of the helices and shows the packing interactions of the x-da arrangement: Residues at x positions stabilize both intrasubunit (solid lines) and intersubunit (dashed lines) interactions; the critical a and d positions (dark gray) contribute to intrasubunit interactions; the less critical a and d positions (light gray) contribute to intersubunit interactions. Two adjacent packing layers are depicted: black interaction lines for the upper layer; gray interaction lines for the lower layer. The residue interactions of the four bundle-packing layers are indicated above the AS1 and AS2 sequences. Amino acid replacements at each AS1 and AS2 residue that abrogate Tsr function are listed in single-letter designation; not all possible replacements were obtained at each position (Zhou et al., 2009). Underlined amino acid replacements cause dominant functional defects (Zhou et al., 2009). Black circles to the left of some replacements indicate lesions that jam the function of wild-type Tar receptors; larger circles denote extremely potent jammers (Zhou et al., 2009). White circles indicate mutant receptors that regain function in the presence of wild-type Tar receptors (Zhou et al., 2009). The histograms illustrate typical flagellar rotation patterns for each mutant output class, as defined in Experimental procedures. The heights of the histogram bars indicate the percentage of rotating cells in each of five categories, from exclusively CCW on the left to exclusively CW on the right. Host strains for the mutant Tsr plasmids were adaptation-deficient [Δ(cheRB)] (dark gray bars) and adaptation-proficient [(cheRB)+] (light gray bars). This shading convention reflects the relative modification state (Q residues or methylated E residues) of wild-type receptors in the two hosts (darker represents higher modification state) and is used for rotation patterns in subsequent figures.