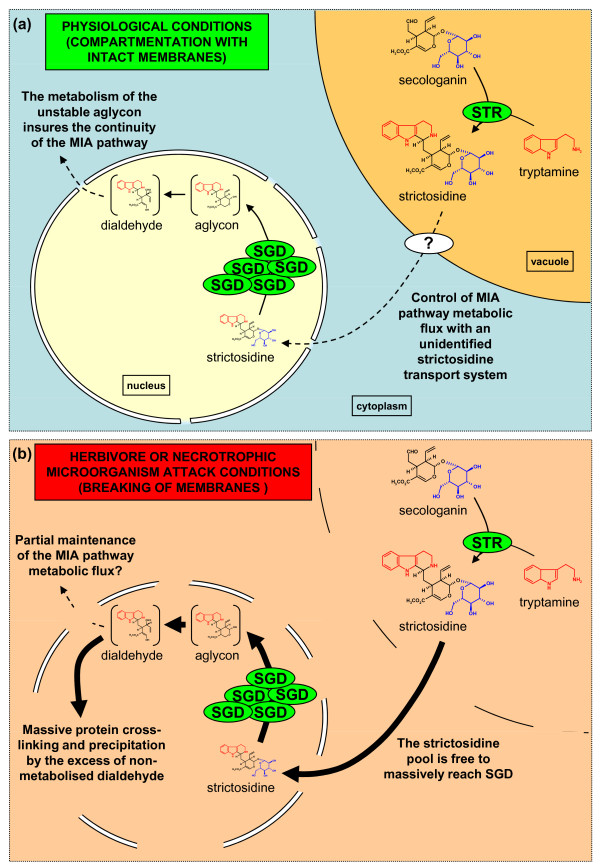
Figure 11.
Working model showing the physiological relevance and the potential plant-defence implications of the vacuole-to-nucleus strictosidine activation. (a) The subcellular compartmentation of STR and SGD is displayed illustrating that an unknown transportation system of strictosidine across the tonoplast (labelled with "?") constitutes a potential important rate limiting step for the flux of MIA biosynthetic pathway. In C. roseus, this part of the pathway is specifically localised to the epidermis of aerial organs. According to the drastic consequences of the CrSGD-mediated massive strictosidine activation (demonstrated by the in vitro experience), this potential rate limiting step appears as a mean for the cells to control the rate of formation of toxic dialdehyde following the deglucosylation of strictosidine by SGD in the nucleus in relation with the metabolic capacity of the next MIA biosynthetic enzymes. (b) In the case of herbivore feeding or necrotrophic pathogen attack, this subcellular compartmentation may be mechanically or enzymatically disrupted. As a consequence, the massive deglucosylation of the strictosidine pool by highly stable SGD aggregates leads to the overproduction of the dialdehyde form to such a level that the MIA biosynthetic machinery is not fully able to take in charge. Massive protein cross-linking and precipitation could therefore be a potential mean for the plant to deter the herbivores and/or the necrotrophic microorganism from their feeding habit.