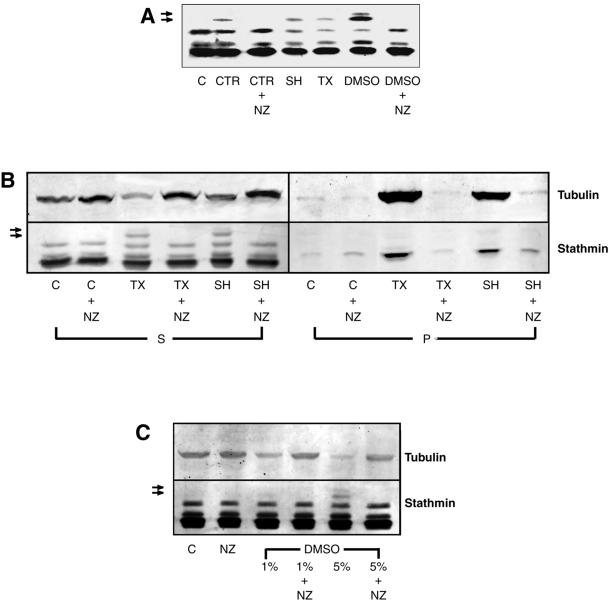
Figure 1.
MT assembly induces stathmin/Op18 hyperphosphorylation in mitotic Xenopus egg extracts. (A) Stathmin/Op18 phosphorylation pattern (probed with antistathmin/Op18 serum I) in low-speed mitotic Xenopus egg extracts in the absence (C, control) or presence of MT-nucleating structures (CTR, somatic centrosomes, 2 × 103/μl extract; SH, sperm heads, 2 × 103/μl extract) or assembly-promoting agents (TX, paclitaxel, 0.1 μM; DMSO, 5%). Nocodazole (NZ, 10 μM) was added in some conditions to prevent MT assembly. MT-dependent hyperphosphorylated forms of stathmin/Op18 are indicated by arrows. (B) Stathmin/Op18 phosphorylation and tubulin distribution in supernatant (S) and pellet (P) of high-speed mitotic Xenopus egg extract after MT polymerization. In all cases, comparative aliquots of both fractions were separated on 12% polyacrylamide gels, and further processed for immunodetection with anti-stathmin/Op18 and anti-tubulin antibodies (MT-dependent hyperphosphorylated forms of stathmin/Op18 are indicated by arrows). (C) Stathmin/Op18 hyperphosphorylation pattern and tubulin content of high-speed extract supernatant in which 1 or 5% DMSO was added. (MT-dependent hyperphosphorylated forms of stathmin/Op18 are indicated by arrows.) In each case, no stathmin/Op18 hyperphosphorylation was detected in nocodazole-containing conditions, even with longer revelation. The patterns shown are representative for each series of at least three experiments leading to similar results.