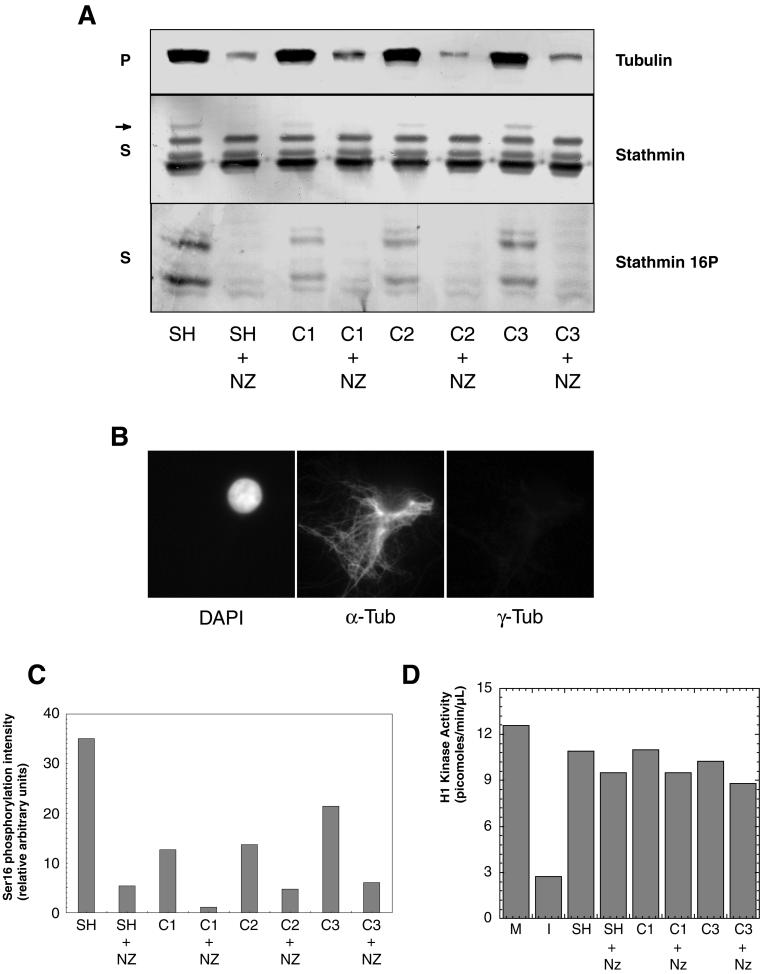
Figure 8.
Nuclei induce stathmin/Op18 hyperphosphorylation through MT assembly. (A) Stathmin/Op18 phosphorylation pattern in supernatants (S) and tubulin distribution in pellets (P) of high-speed mitotic extracts after addition of sperm heads (SH) or nuclei at various concentrations (C1: 7.5 × 103; C2: 9 × 103; C3: 1.1 × 104 in nuclei/μl), with or without nocodazole (NZ). The MT-dependent hyperphosphorylated forms of stathmin/Op18 are indicated by arrows (the upper band is visible on the original Western blot). (B) Immunofluorescence analysis of citric acid nuclei added to high-speed mitotic Xenopus egg extracts. Nuclei are visualized with DAPI and MTs are assembled around chromatin in the absence of centrosomes. α-tub, anti-α-tubulin antibody; γ-tub, anti-γ-tubulin antibody. (C) Graph showing the intensity of the bands corresponding to phosphorylation on stathmin/Op18 Ser 16 in A (stathmin 16P). Results are expressed for each lane as a ratio of phosphorylation on Ser 16 (stathmin 16P) to total stathmin/Op18 (stathmin), which remains constant throughout the experiment and thus represents an internal control. (D) H1 kinase activity quantitation of extracts to which sperm heads or nuclei was added, in the absence or presence of nocodazole (NZ). In neither case was the original mitotic extract (M) shifted to interphase, as happens when calcium (0.4 mM) is added (I).