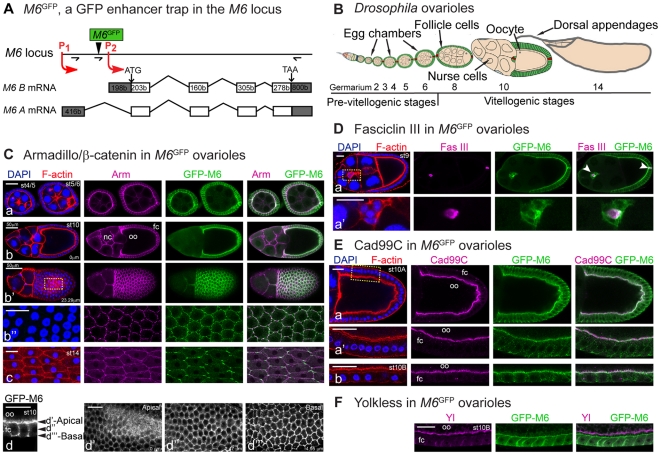
Figure 4. GFP-M6 is restricted to the membrane of the follicular epithelium.
(A) M6 localization was investigated using M6 GFP (M6CA06602). The P-element is indicated (black arrowhead). RT-PCR primers are indicated by arrows (see Table S1). All M6 isoforms derived from P1 are tagged to GFP at the N-terminus (Zappia et al., unpublished data). (B) Schematic diagram of the Drosophila ovariole (adapted from [53]). A monolayer of follicle cells (green) derived from somatic stem cells surround the germ-line cyst (yellow). Polar cells are labeled in red. Developmental stages are indicated (2 to 14). (C–F) Follicular markers were used to analyze GFP-M6 localization. Throughout this work representative images are shown and numbers in each panel indicate developmental stages determined by F-actin (Phallodin, red) and nuclei (DAPI, blue) staining (left panels). The anterior-posterior axis is oriented left to right. (C) Armadillo/β-catenin (magenta) labels the membrane of follicle and nurse cells. GFP-M6 is localized to the membrane of the FE (green). (a–c) Egg chambers from early (a), mid (b-b″) and late (c) oogenesis. Apical/basal (b, 0 µm) and top (b′, 23.29 µm) views of a st10 egg chamber are shown. (b″) A magnified view of the area indicated in b′. Note the change in GFP-M6 distribution within the membrane at later stages (b″ and c). (d-d′″) Apical/basal view of st10 follicle cells (d), and sections corresponding to an apical (d′, 0 µm), middle (d″, 2.47 µm) and basal (d′″, 4.95 µm) membrane domains. (D) GFP-M6 localizes to polar and border cells (white arrowheads), the former labeled with anti-Fasciclin III (magenta); (a′) is a magnified view of the region indicated in a. (E) GFP-M6 localizes to the microvilli extending from the FC apical membrane, visualized with anti-Cad99C antibody (magenta); apical/basal views of the FC from st10A and st10B are shown (a-a′ and b, respectively), along with a magnified view of the indicated region (a′). (F) GFP-M6 is not expressed in the oocyte cortex, stained with anti-Yolkless antibody (magenta). Images (Cb″ and Ea′-b) were processed with the Adaptive PSF Deconvolution method. Scale bar is 20 µm, unless otherwise indicated.