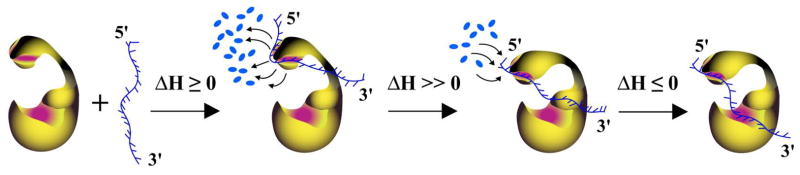
Figure 10.
Schematic model of the pol β-ssDNA complex formation. In the initial complex, the enzyme engages only the 8-kDa domain in a process accompanied by the release of the large number, ~16 – 19, water molecules and characterized by zero or a small positive ΔHo. The entire potential DNA binding subsite of the 8-kDa domain is involved in interactions with the nucleic acid. Transition to the (pol β)5 binding mode includes reorientation of the 8 -kDa domain with respect to the 31-kDa domain. The process is accompanied by the net uptake of ~ 10 water molecules by the 8-kDa domain and a large positive ΔHo. The final engagement of the total DNA-binding site in forming the (pol β)16 binding mode, involves engagement of the DNA -binding subsite on the 31-kDa domain in interactions with the nucleic acid, without additional water release and accompanied by a small negative ΔHo (details in text).