Figure 4.
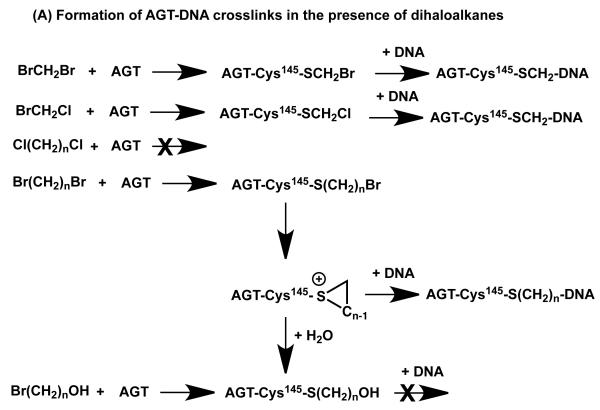
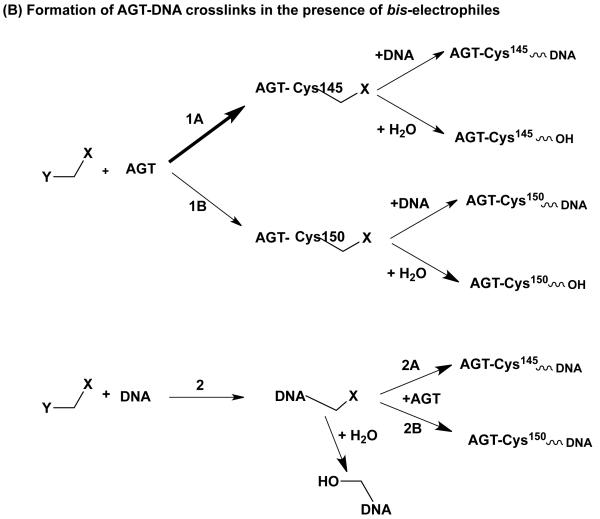
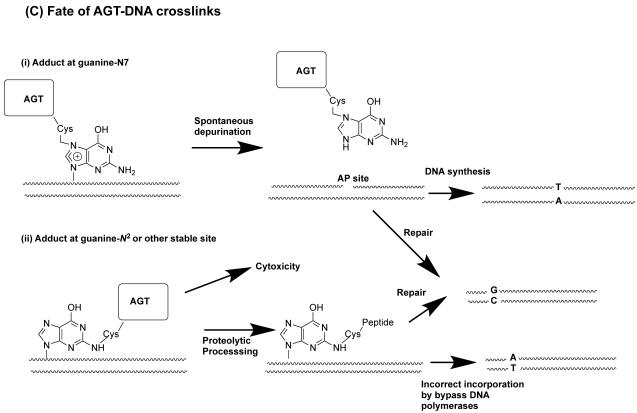
Formation of DNA-AGT crosslinks in the presence of dihaloalkanes and other bis-electrophiles. (A) Reactions of dihaloalkanes to generate crosslinks at Cys145. (B) Reactions of bis-electrophiles to generate crosslinks at Cys145 and Cys150. The chemical can react directly with AGT at either Cys145 or Cys150 as shown in 1A and 1B respectively. Further reaction with DNA then forms the AGT-DNA crosslink. The bis-electrophile may also react with DNA directly to generate a reactive species that can then react with AGT to produce the AGT-DNA crosslink at either Cys residue as shown in 2A and 2B. (C) The AGT-crosslinks can cause mutations or cytoxicity. (i) Attachment at the guanine N7 position generates an unstable linkage that can spontaneously depurinate to form an AP site which on DNA replication can then lead to the observed G:C to T:A transversions. (ii) More stable adducts, possibly at the guanine N2, although this has not been characterized, can be processed and copied by bypass polymerases to lead to the observed G:C to A:T transitions.