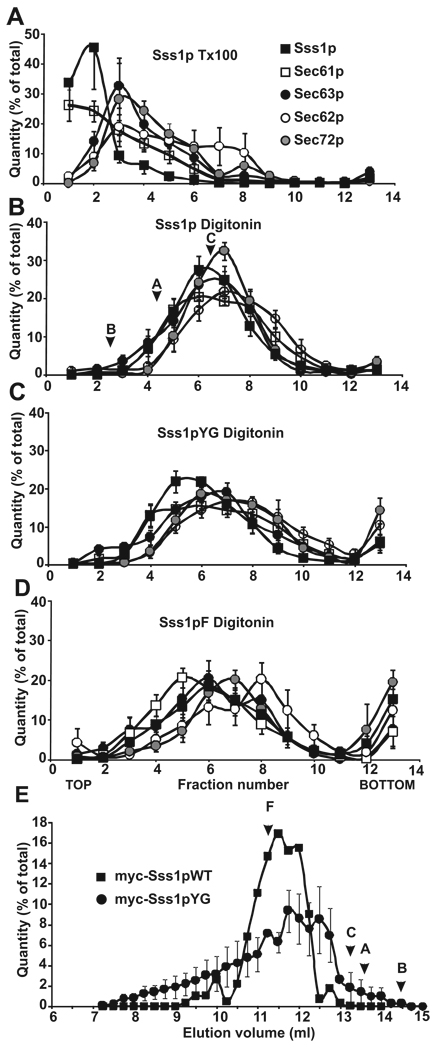
Figure 4. Sec complexes containing tail-anchor mutants are intact but altered when assayed by sucrose density gradient centrifugation.
Post-nuclear membranes derived from strains expressing Sss1pWT, Sss1pYG, or Sss1pF were solubilized in 1% Triton X-100 (A) or 2.5% digitonin (B–D) and complexes were resolved by sucrose gradient centrifugation (linear gradient of 25–35% sucrose). Gradients were divided into 13 fractions, acid precipitated, and analyzed by SDS-PAGE and immunoblotting with antibodies to Sec63p (black circles), Sec62p (white circles), Sec72p (grey circles), Sec61p (white squares), and Sss1p (black squares). Protein bands in each fraction were quantified, expressed as a percent of the total, and plotted by fraction number. The sedimentation positions of molecular weight standards run in parallel (digitonin only) are marked: B, Bovine Serum Albumin (67 kDa), A, Aldolase (158 kDa), C, Catalase (232 kDa). (E) Post-nuclear membranes derived from yeast expressing myc- tagged Sss1pWT (black squares) and myc-tagged Sss1pYG (black circles) were salt extracted, solubilized in 2.5% digitonin buffer, and Sec complexes were resolved by separation on a Sephadex 200 FPLC column. Samples eluting from 7 to 15 ml were collected in 0.5ml fractions, precipitated, and analyzed as in (A). Immunoblots were probed with antibodies to myc, quantified, expressed as a percent of the total, and plotted by elution volume. Elution positions of molecular weight standards are marked: F, Ferritin (440 kDa); C, Catalase (232 kDa); A, Aldolase (158 kDa); B, BSA (67 kDa). Panels A–D represent averages of 6 experiments.