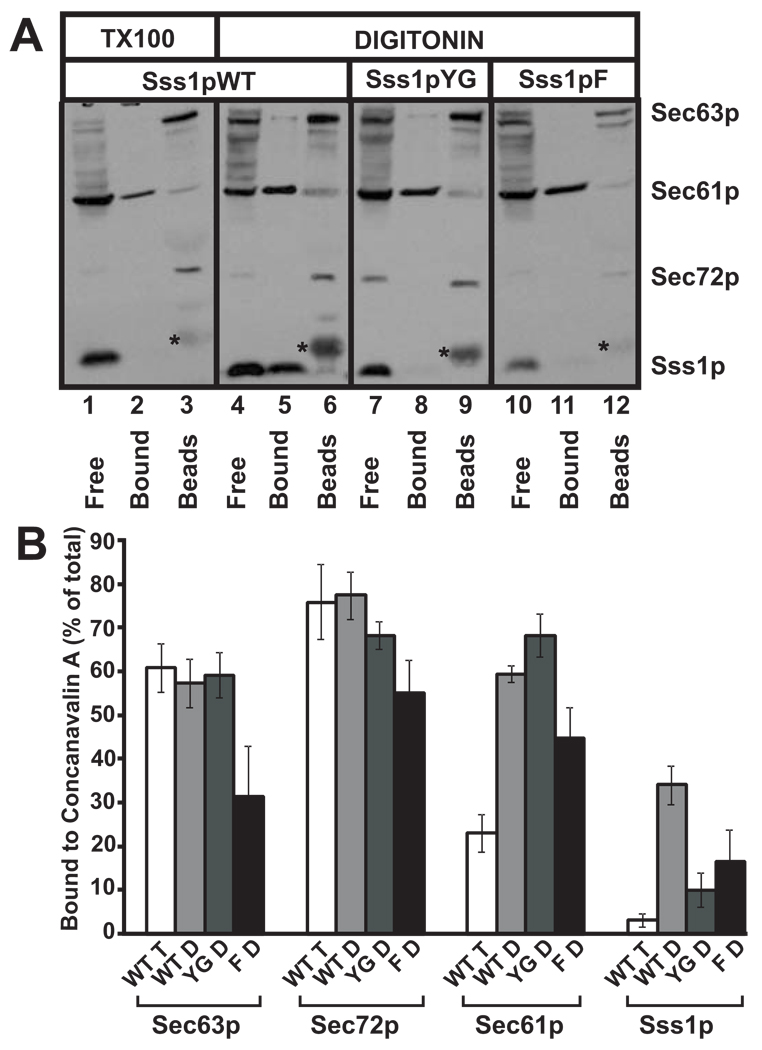
Figure 5. Digitonin-soluble Sec complexes prepared from membranes from mutant Sss1p strains are unstable when assayed by binding to Concanavalin A Sepharose.
(A) Post-nuclear membranes derived from yeast expressing Sss1pWT, Sss1pYG, and Sss1pF were salt extracted, washed several times to remove EDTA, solubilized in either 2.5% digitonin or 1% Triton X-100 (wild type only), and pre-cleared by centrifugation to remove insoluble components. Soluble Sec complexes were bound to Concanavalin A Sepharose (Con A beads) which were separated from free Sec61 complexes by low speed centrifugation. Bound Sec61 complexes forming part of the Sec complex were then eluted from the Con A beads with 1% Triton X-100 and high salt. Samples were either acid precipitated or had loading buffer added (Con A beads) and were analyzed by SDS-PAGE and immunoblotting with antibodies to Sec63p, Sec61p, Sec72p, and Sss1p. Free, fraction of detergent-soluble proteins not bound to Concanavalin-A; Bound, proteins bound to Concanavalin-A and eluted with 1% Triton X-100 and 500mM NaCl; Beads, proteins still bound to Concanavalin-A after elution with 1% Triton and 500mM NaCl; * contaminant eluted from beads. (B) Experiments performed as described in (A) were quantified and expressed as percent bound to Con A for each protein indicated. WT T, Sss1pWT-derived post-nuclear membranes solubilized in 1% Triton X-100; WT D, YG D, F D, digitonin-solubilized post-nuclear membranes derived from strains expressing Sss1pWT, Sss1pYG, and Sss1pF, respectively.