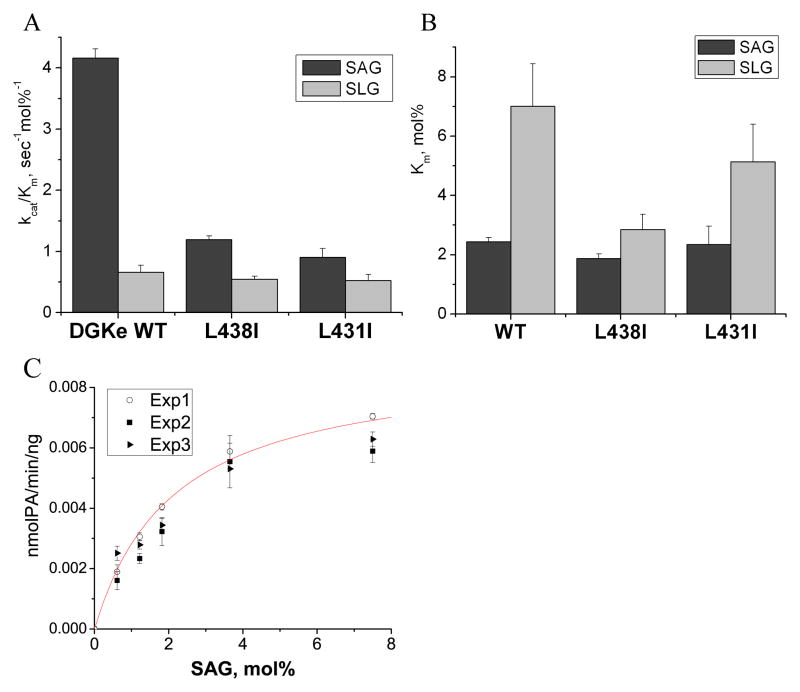
Figure 6.
Graphic presentation of the kinetic parameters for FLAG-DGKε WT and its L438I and L431I mutants. Results are presented as the mean ± S.D. A. Results show that kcat/Km is greatly affected (about 4-fold decrease) by L438I and L431I mutations of DGKε for SAG, but not SLG substrate. B. Comparison of Km parameters shows that L438I and L431I mutations of DGKε slightly affect the substrate affinity of the enzyme, decreasing the preference for SAG over SLG. C. An example of kinetic data of 3 independent experiments (Exp1, Exp2 and Exp3) is shown for FLAG-DGKε WT with SAG as a substrate. Every experiment is performed in triplicate. A nonlinear regression curve fitting Exp1 data is shown in red.