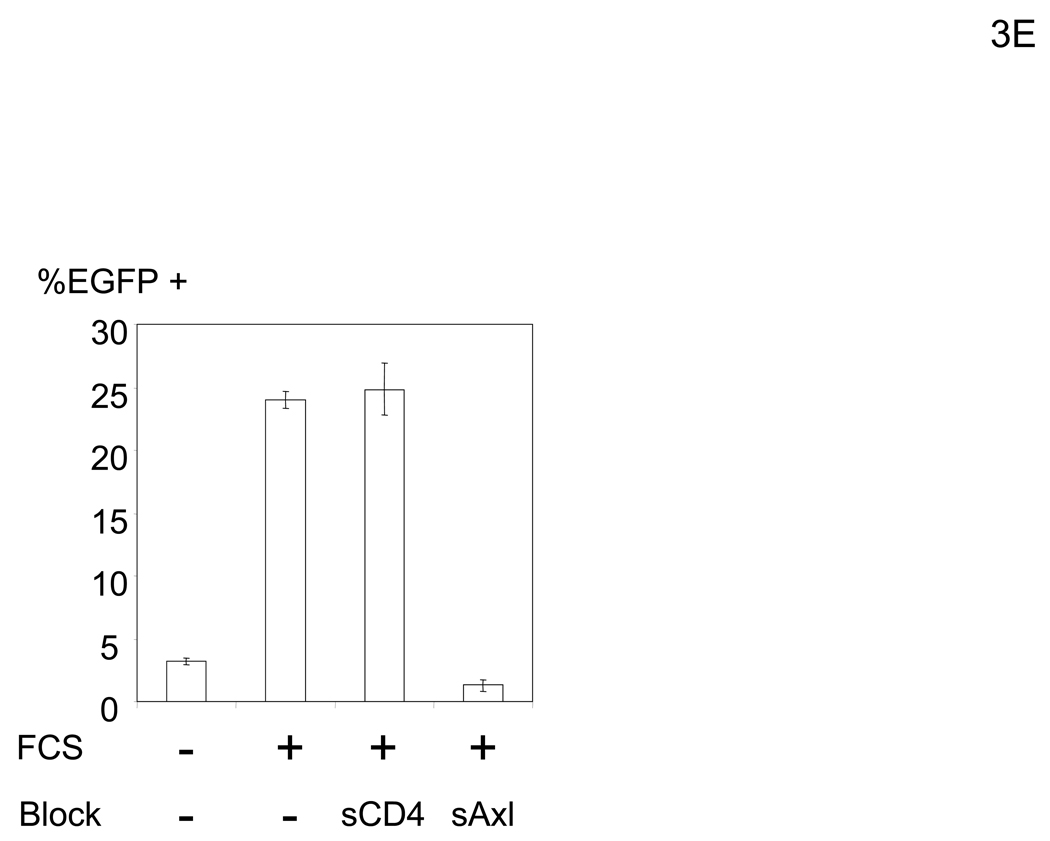
Figure 3.
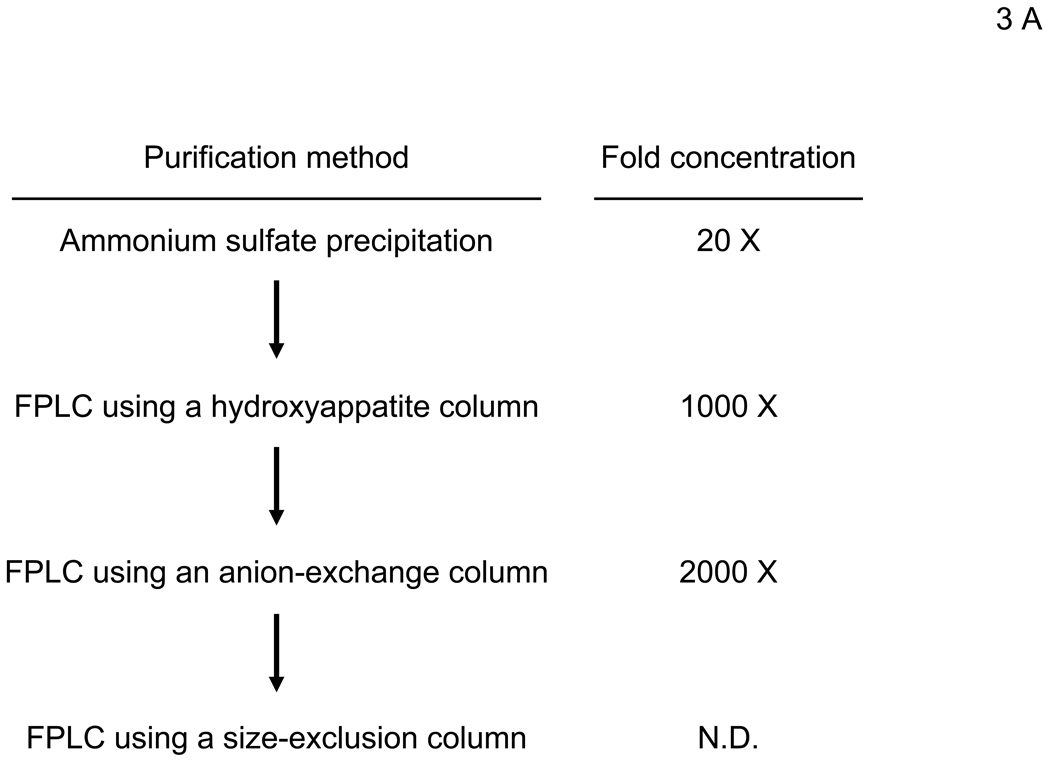
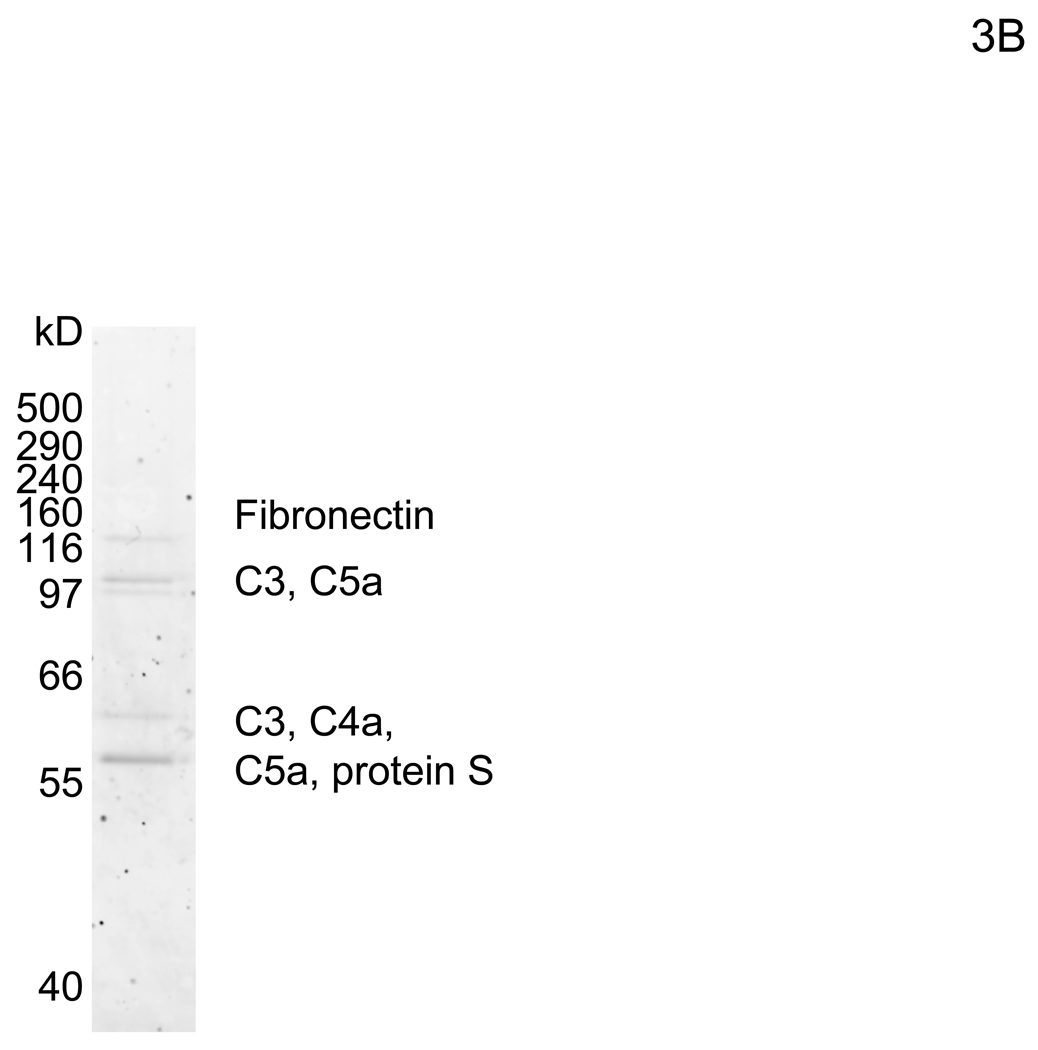
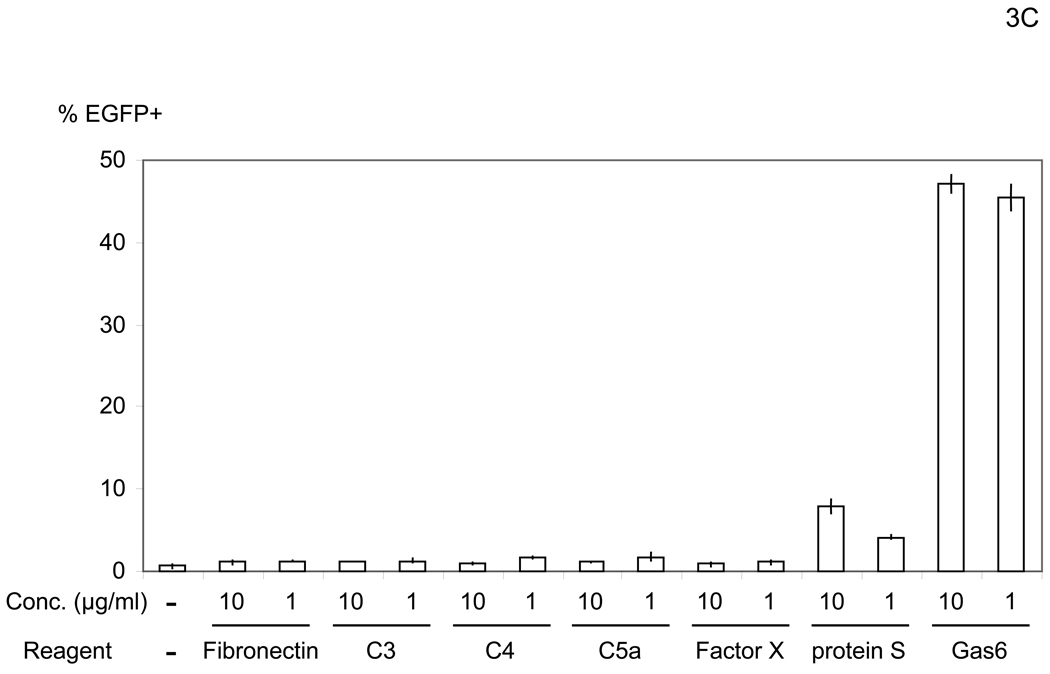
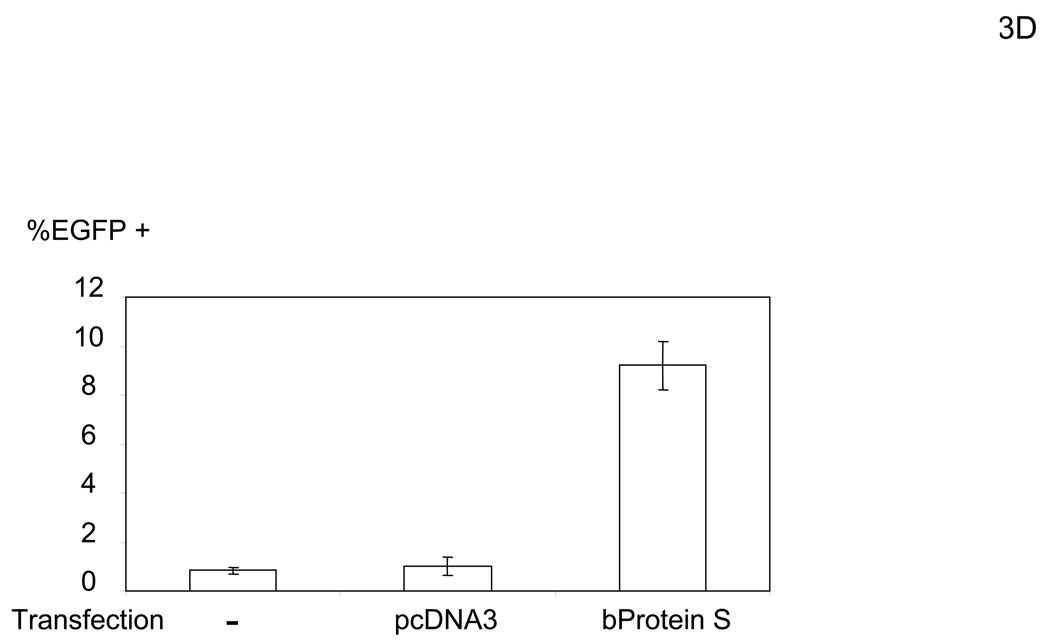
The bridging factor in FCS is bProtein S. (A) FCS was fractionated by precipitation with various concentrations of ammonium sulfate (5% increments). The proteins precipitated with 33–38% ammonium sulfate had the highest enhancing activity/mg of protein and was used for subsequent purification by FPLC using hydroxyappatite, anion-exchange, and size exclusion columns. Each fraction was tested for enhancement of transduction of HMVEC with the 2.2 1L1L pseudotype. The fraction(s) showing the highest enhancing activity were used for subsequent purification. The fold concentration of the purified fractions was calculated by the following formula: Protein concentration of FCS necessary for 10-fold enhancement of HMVEC transduction with 2.2 1L1L pseudotype divided by protein concentration of each fraction necessary for the same enhancing activity. Because the protein concentration of the fraction purified by size-exclusion chromatography was too small to measure, fold concentration could not be determined, and is shown as N.D. (B) The size-exclusion chromatography fraction which had the highest enhancing activity was subjected to SDS-PAGE and SyproRuby staining. The protein bands were then subjected to protein identification by mass spectrometry. Since the two bands migrated around 97 kD, we identified the proteins in these two bands together. (C) HMVEC were incubated with the human homologues of the proteins identified in the purified fraction, followed by transduction with the 2.2 1L1L pseudotype (100 ng p24/ml). (D) HMVEC were incubated with the supernatant of 293T cells transfected with an expression vector for bProtein S or control plasmid (pcDNA3) or serum-free medium, followed by transduction with the 2.2 1L1L pseudotype (100 ng p24/ml). (E) 10% FCS was incubated with sAxl or sCD4 (25 µg/ml) in PBS (+) for 1 hour and used to incubate HMVEC for 4 hours. The cells were then infected with the 2.2 1L1L pseudotype (100 ng p24/ml).