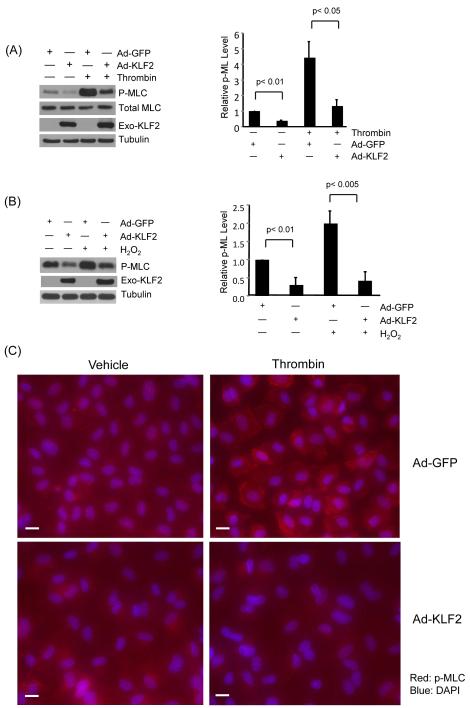
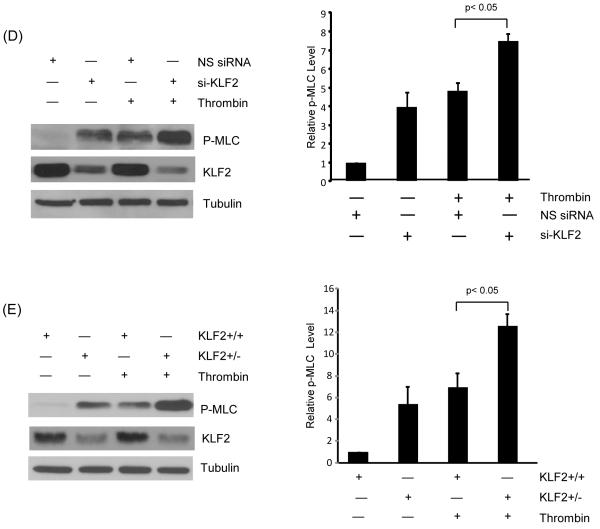
Figure 5.
KLF2 inhibits phosphorylation of MLC in response to agonists. (A) HUVEC were infected with Ad-GFP and KLF2 adenovirus for 24 hrs, cells were then treated with thrombin (1U/ml) for 15 and 30 minutes, proteins were then extracted. Western blot analysis reveals attenuation of MLC phosphorylation in KLF2 overexpressed cells. Representative blot of three independent experiments is shown. (B) HUVEC were infected as above, followed by treatment with hydrogen peroxide for 30 minutes and 60 minute, total protein were isolated and subjected to western blot. Representative blots of three independent experiments are shown. (C) HUVEC infected with Ad-GFP and KLF2 adenovirus for 24 hrs were treated with thrombin (1U/ml) for 15 minutes, then immunostaining with phospho-MLC antibody was performed. Representative pictures of three independent experiments are shown. Scale bar, 50 μm. (D) HUVEC were transfected with non specific siRNA and siKLF2 using siPortAmine. After 48 hrs the cells were treated with thrombin (1U/ml) for 1 minute and total protein harvested using RIPA buffer. Western blot analysis revealed increased phosphorylation of MLC in siKLF2 transfected cells when compared to non specific siRNA transfected cells. (E) Primary lung microvascular endothelial cells were isolated from KLF2+/+ and KLF2+/− mice, they were then treated with mouse thrombin for 1 minute, proteins were extracted and western blot performed. A significant increase of MLC phosphorylation was observed in KLF2+/− ECs than that of KLF2+/+ ECs. A representative blot of three independent experiments is shown.