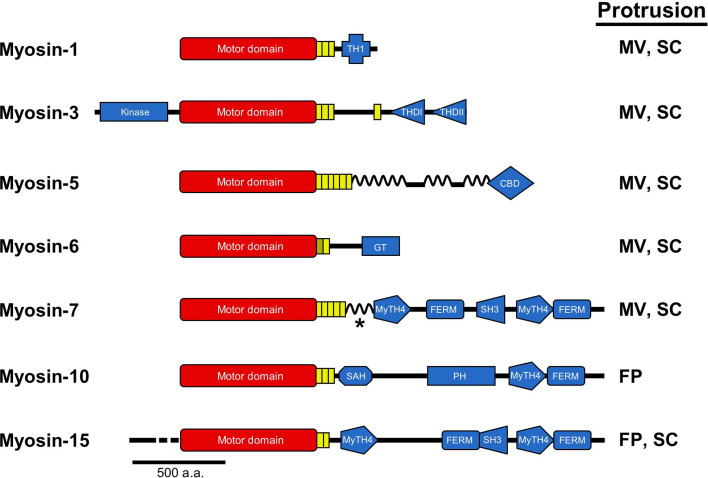
Fig. 3.
Myosins that reside in PAB-supported protrusions. Bar diagrams representing the primary structures of the seven different myosins discussed in this review are drawn along with an indication of the protrusion(s) that each motor occupies (FP filopodia, MV microvilli, SC stereocilia). All myosins possess N-terminal motor domains (red) coupled to structurally diverse C-terminal tail domains (blue). Myosin-1 contains a tail homology domain I (THI), which directs binding to membrane lipids. Myosin-3 is unique among myosins in that it possesses an N-terminal kinase domain. Tail homology domains I (THDI) and II (THDII) are also shown; the former binds to espin 1, while the latter may mediate direct binding to actin. Myosin-5 is a dimeric and processive motor with coiled-coil forming regions throughout its tail and a cargo-binding domain at the C-terminus. Myosin-6, the sole minus-end directed motor, is depicted with a cargo-binding globular tail domain (GT), which also mediates dimerization. Myosins-7, 10 and 15 are MyTH4-FERM domain myosins containing at least one MyTH4-FERM domain in their tails. These domains are known to mediate binding to a number of important proteins and lipids: the FERM domain of myosin-7a binds the transmembrane protein vezatin and thus links it to the cadherin/α-catenin complex; the MyTH4 domain of myosin-10 binds to microtubules, whereas the FERM domain binds to the cytoplasmic NPXY domain of β-integrins; the FERM domain of myosin-15 binds to the third PDZ domain of Whirlin, its primary cargo. In the case of myosin-7, the asterisk represents a coiled-coil domain that may facilitate dimerization in myosin-7a. Myosin-10 also has a non-dimerizing stable alpha helix (SAH) and PH domain that mediates interactions with PIP3