Abstract
The Na+/Ca2+ exchanger (NCX1) is crucial in the regulation of [Ca2+]i in the cardiac myocyte. The exchanger is upregulated in cardiac hypertrophy, ischemia, and failure. This upregulation can have an effect on Ca2+ transients and possibly contribute to diastolic dysfunction and an increased risk of arrhythmias. Studies from both in vivo and in vitro model systems have provided an initial skeleton of the potential signaling pathways that regulate the exchanger during development, growth, and hypertrophy. The Ncx1 gene is upregulated in response to α-adrenergic stimulation. We have shown that this is via p38α activation of transcription factors binding to the Ncx1 promotor at the −80 CArG element. Interestingly, most of the elements, including the CArG element, which we have demonstrated to be important for regulation of Ncx1 expression are in the proximal 184 bp of the promotor. Using a transgenic mouse, we have shown that the proximal 184 bp is sufficient for expression of reporter genes in adult cardiomyocytes and for the correct spatiotemporal pattern of Ncx1 expression in development but not for upregulation in response to pressure overload.
Keywords: Na+/Ca2+ exchanger, NCX1, cardiac hypertrophy, regulation of gene expression, signal transduction, transgenic mice
INTRODUCTION
The heart is very dynamic and adapts to changes in hemodynamic demands by the enlargement of the ventricle via individual cardiomyocyte hypertrophy. The cardiac hypertrophic response is characterized by an increase in cardiac mass, protein content, and size of the individual cardiocyte. Initially, this results in increased myofilaments and improved contractile function. But, if the pathological stimulus is prolonged or sufficiently severe, and the increase in mass is insufficient to normalize ventricular wall stress, decompensated hypertrophy or heart failure will occur. These stimuli are often initiated by chronic hypertension, myocardial infarction, or ischemia associated with coronary artery disease. One of the features of the failing heart is a prolonged action potential and depressed contractility. In many models of heart disease and failure, the expression and activity of the Ca2+ sequestering sarcoplasmic reticulum (SR) Ca2+-ATPase is decreased, and/or the activity and protein level of Na+/Ca2+ exchanger (NCX1) is increased.1–4 The exchanger catalyzes the electrogenic exchange of Ca2+ and Na+ across the plasma membrane in either the Ca2+-influx or Ca2+-efflux mode. NCX1 is one of the essential regulators of Ca2+ homeostasis within cardiomyocytes and changes in its activity can affect contractility. The exchanger is upregulated at the transcriptional level in cardiac hypertrophy and failure.1,5–8 Increased exchanger activity leads to increased Ca2+ extrusion and acts to preserve low diastolic Ca2+ levels, which may compensate in part for depressed SR Ca2+-ATPase function. However, this adaptation has been demonstrated to have a number of deleterious consequences. First, in the failing heart, peak systolic Ca2+ is decreased because a higher percentage of [Ca2+]i is being removed by NCX1, rather than returning to the SR.9 In addition to the increase in NCX1 expression and activity, and reduced SR Ca2+-ATPase function, Marx et al.10 have shown that the ryanodine receptor is hyperphosphorylated in the failing heart. This results in increased overall Ca2+ release from the channel and has also been shown to contribute to a persistent unloading of SR Ca2+ stores.
Second, changes in Ncx1 expression and/or activity can contribute to aberrant contractile function. Because the exchanger is an electrogenic transporter, the increase in Ca2+ translocation across the plasma membrane results in greater risk of an inappropriately triggered depolarization (delayed after depolarization, DAD) before the relaxation cycle has completed.3,11 This can cause arrhythmia and sudden death, a common cause of death from heart disease. Another potential problem arises from the lower levels of cytosolic Ca2+ in combination with prolonged action potential duration, which can promote reverse mode, Ca2+ in Na+ out, activity. This would not only slow the rate of decay of the Ca2+ transient and relaxation, but also contribute to possible arrhythmogenesis and diastolic dysfunction.12 Finally, in hearts that have been exposed to ischemia or hypoxia, reoxygenation can result in serious injury due to hyper-contracture caused by spontaneous Ca2+ oscillations.13 Several studies have shown that these oscillations are primarily elicited by reverse mode activity of the NCX1.14–16 There is still a great deal more to be understood, but clearly, changes in exchanger expression are important to cardiac function. Therefore, we have begun to identify the molecular pathways and transcription factors and elements that are responsible for the upregulation of the exchanger gene in cardiac hypertrophy and failure.
There are multiple tissue-specific variants of Ncx1 resulting from alternative promotor usage (H1, K1, and Br1) and alternative splicing.17–19 Using transgenic models we demonstrated that the 1831Ncx1 H1 promotor was sufficient for driving the normal spatiotemporal pattern of NCX1 expression in cardiac development.17 Importantly, at later fetal stages (E14 and older), and in the adult the 1831Ncx1 H1 promotor-driven reporter gene expression remains heart specific when the normal heart-restricted pattern of the endogenous NCX1 is no longer present.20 Koban et al. showed cardiac-specific expression of reporter genes using either 2.1 kb or 2.6 kb rat Ncx1 H1 promotor.21 Expression was first detected at E7.5 and remained cardiac specific in the adult animal. Interestingly, here reporter gene expression was diminished with age. Importantly, we demonstrated that the feline 1831Ncx1 H1 promotor is sufficient for the upregulation of Ncx1 in response to pressure overload in transgenic mice.17
p38 ACTIVITY IS IMPORTANT FOR MEDIATING Ncx1 EXPRESSION
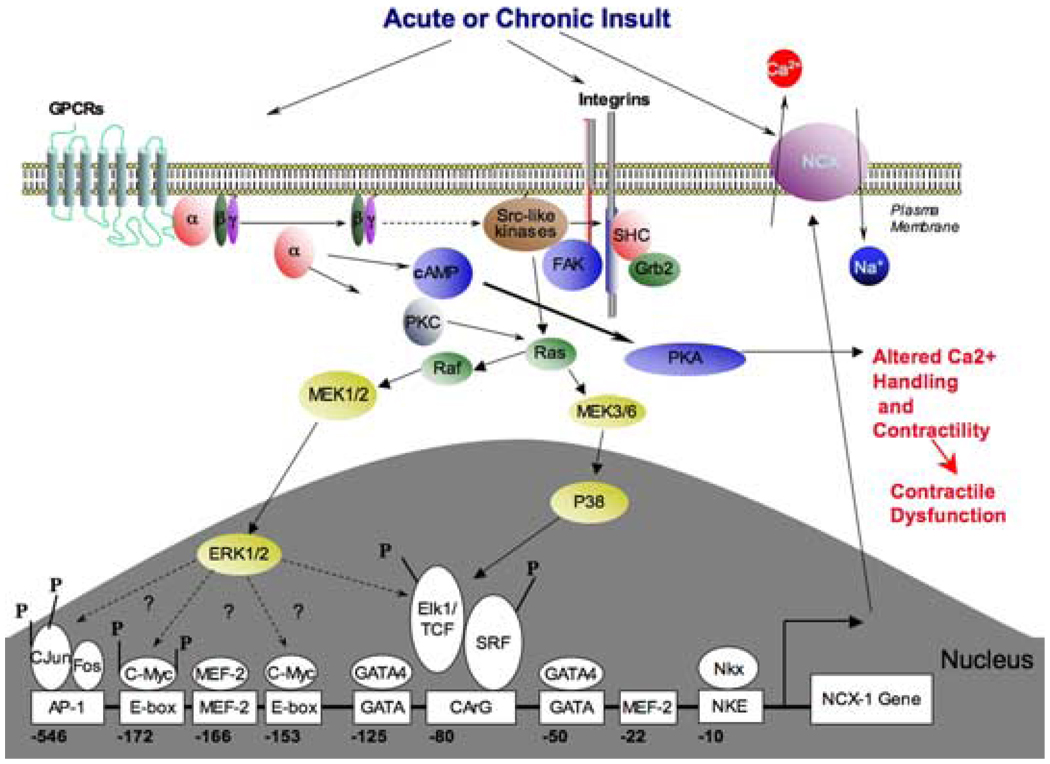
Several signaling pathways, including those activated by G protein–coupled receptor (GPCR) agonists, integrins, nonreceptor protein tyrosine kinases, protein kinase C, intracellular Ca2+, and calcineurin are implicated in the initiation and maintenance of hypertrophy. The mitogen-activated protein kinases (MAPK), which transduce extracellular signals into the nucleus, are induced by hypertrophic stimuli. Importantly, their activation can initiate a hypertrophic response. p38 MAPK is of distinct interest since it has been shown to play a possible role in cardiocyte growth,22 proapoptotic23 or antiapoptotic24 pathways. In vitro studies in neonatal cardiomyocytes show that activation of p38 induces expression of atrial natriuretic peptide22,23,25 and inhibition of p38 reduced agonist-induced brain natriuretic peptide promotor activity.26,27 Activation of p38 has been shown to mediate a negative inotropic effect, contribute to loss of contractility, and increase myocardial stiffness.28 Andrews et al. have also shown that p38 can mediate the downregulation of SERCA2, which resulted in altered [Ca2+]i regulation.29 In addition to regulating SERCA, p38 also plays a role in mediating Ncx1 expression.30–32 We showed that the p38 α/β inhibitor SB202190 inhibits 50% of Ncx1 upregulation mediated by α-adrenergic stimulation.32 Overexpression of a dn p38 in adult cardiomyocytes also leads to a 50% decrease in α-adrenergic stimulation of Ncx1-promotor-driven reporter gene as well as endogenous Ncx1 transcript. Our results clearly showed that p38 contributed to the α-adrenergic-mediated Ncx1 upregulation. The MEK-1 and -2 inhibitor U0126 inhibits approximately 35% of the PE-stimulated Ncx1 upregulation. Therefore, p38 and ERK-1 and -2 together mediate the majority of the α-adrenergic stimulation of the Ncx1 promotor in adult cardiomyocytes (FIG. 1). In order to determine if activated p38 is sufficient for induction of the Ncx1 promotor, we overexpressed the constitutively active forms of the p38 upstream kinases, MKK3 and MKK6. Overexpression of either MKK3 or MKK6 resulted in upregulation of the Ncx1 promotor-driven reporter gene.32 p38α, p38δ, and p38γ have been detected in the heart with p38α as the predominant isoform.33 p38β has not been detected at the mRNA or protein level in human,33 rat,34 or mouse28 heart. SB202190 completely prevented NCX1 upregulation in response to overexpression of MKK3bE or MKK6bE. p38α is the only p38 isoform activated by MKK3bE, which is also inhibited by SB202190. Therefore, activation of p38 is not only critical for a portion of the α-adrenergic-stimulated Ncx1 upregulation, it is sufficient for Ncx1 upregulation and it appears that this is mediated chiefly via p38α.
FIGURE 1.
Diagram of the signaling pathways and transcription elements on the proximal Ncx1 promotor important for the regulation of exchanger expression in the adult cardiocyte.
THE Ncx1-80 CArG ELEMENT MEDIATES p38-STIMULATED UPREGULATION OF THE EXCHANGER
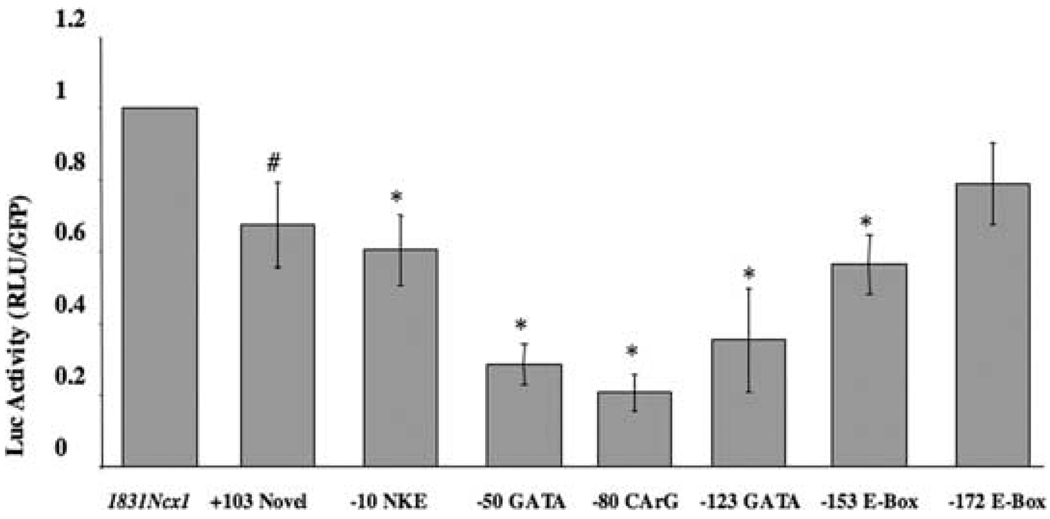
The Ncx1 promotor contains several consensus sequences for a number of potential DNA-binding factors, which we have shown to be important in regulating Ncx1 expression in neonatal6 and adult cardiomyocytes. These include two GATA elements, two MEF2 elements, two E-Box elements, a CArG element, and a binding site for Nkx 2.5 (FIG. 1). These elements are also present in the human and rat and interestingly, the first 184 bp of the human35 and feline19 Ncx1 H1 promotors have a 97% identity and the rat36 and feline sequences 92%. In isolated adult cardiocytes the −123 GATA and −50 GATA element resulted in 35% and 29% of wild-type promotor activity respectively (FIG. 2). A point mutation disrupting the −80 CArG element resulted in luciferase activity of less than 20% of the control level.37 In addition to the CArG and GATA elements being important to basal Ncx1 expression in adult cardiomyocytes, the −80 CArG element is required for Ncx1 upregulation in response to p38 activation (see FIG. 1).32 Our study also showed that the −80 CArG element is responsible for part of the α-adrenergic-stimulated upregulation.
FIGURE 2.
Effect of Ncx1 promotor transcriptional element mutations on basal gene expression in adult feline cardiomyocytes. Adult feline cardiomyocytes were infected with the wild-type or mutant Ncx1 promotor luciferase reporter adenoviruses (MOI 1.5). Cells were then incubated for 48 h and lysed in reporter buffer. Luciferase activity was determined relative to green fluorescent protein. All values are averages from four separate cell isolation experiments performed in triplicate. *P < 0.0002 when compared to wild-type promotor and # P < 0.007 when compared to wild-type promotor. (Figure taken with permission from Ref. 37.)
THE PROXIMAL 184 bp OF THE PROMOTOR IS SUFFICIENT FOR EXPRESSION BUT NOT FOR UPREGULATION IN RESPONSE TO PRESSURE OVERLOAD
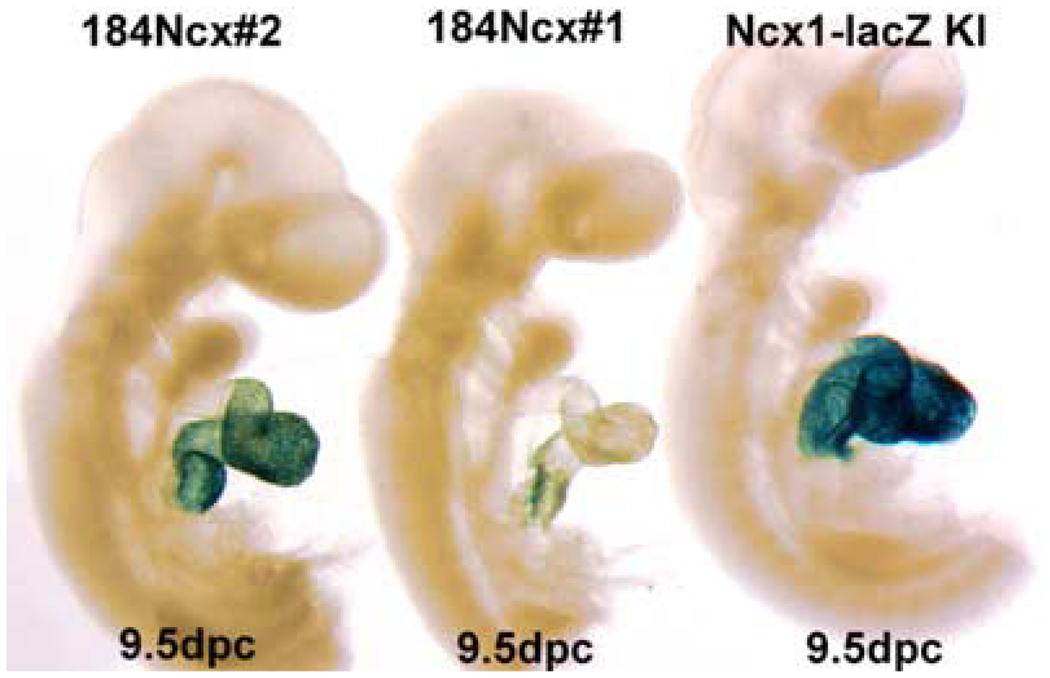
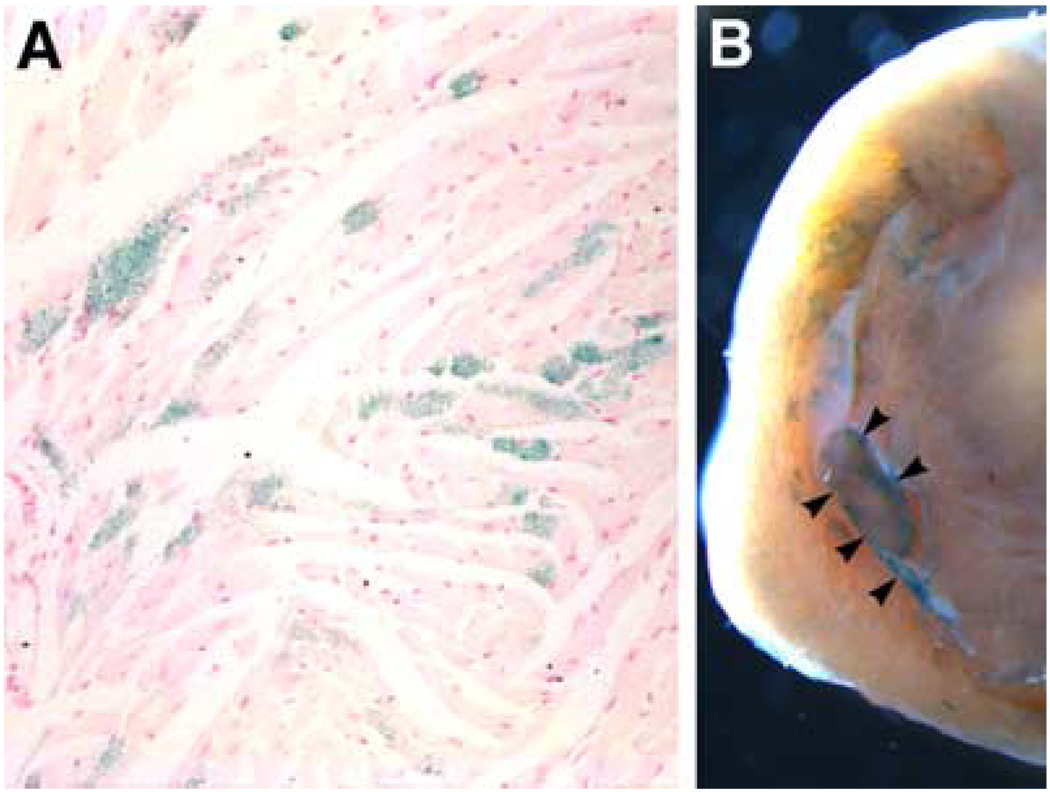
The majority of the consensus sequences for DNA-binding factors, which are important in regulating Ncx1 expression, are found in the proximal 184 bp of the promotor. Our early work demonstrated that the 184Ncx1 promotor is sufficient for driving reporter expression in both neonatal and adult cardiomyocytes. Interestingly, the 184Ncx1 promotor is upregulated in response to α-adrenergic stimulation in neonatal cardiomyocytes but is recalcitrant to α-adrenergic stimulation in adult cardiomyocytes.6,37 Subsequently, Xu et al. demonstrated that the 184Ncx1 promotor fragment could direct LacZ expression to the embryonic heart, with no expression in extracardiac tissues.37 Expression is restricted to cardiomyocytes and is not observed in endothelial or endocardial cushion cells. The penitrance of expression varies between transgenic lines (FIG. 3). But even in lines where most cardiomyocytes express the reporter at 9.5 days (see line 1 FIG. 3) the expression pattern becomes patchy by E14.5 and diminished overall in the newborn37 and in the adult (FIG. 4 A). In addition to the patchy cardiac myocyte expression in the adult, 184Ncx1-β-gal reporter activity was detected in the sinus venosus, and the pulmonary and caval veins, which was never observed in any of the 1831Ncx1 transgenic mouse lines.17 Koban et al. showed for the rat Ncx1 promotors, that expression of the transgene could be detected in the conduction system.21 Interestingly, even though reporter gene expression is extremely patchy in the adult the 184Ncx1-β-gal transgene expression is consistently found within the cardiac myocytes that appeared to be part of the adult conduction system (FIG. 4 B). Positive lacZ staining could be detected in cells surrounding papillary muscles and in the wall of the left ventricle (FIG. 4 B, arrowheads). These data indicate that the 184Ncx1-β-gal transgene contains the enhancer elements sufficient to drive the correct cardiac myocyte-specific expression early in development but that it is missing some of the elements that contribute to the persistence of ubiquitous cardiomyocyte expression and extracardiac expression in the neonate and adult. What appears to be affected is the probability of the expression of the reporter gene in any particular cardiocyte not the level of expression. This probability of expression may be highest in specialized cardiomyocytes that make up the conduction system, though we have not yet confirmed this using a specific marker against specialized conduction system myocytes. Additionally, most of the cells that continue to express the transgene in late development and in the adult appear to be subendocardial rather than subepicardial.
FIGURE 3.
Temporal–spatial detection of endogenous Ncx1lacZ and 184Ncx1 β-galactosidase reporter activity within the mouse cardiovascular system. Both 184Ncx1 and endogenous Ncx1lacZ lacZ expression are present in utero throughout the E9.5 embryonic heart, and absent from the rest of the embryo and extraembryonic tissues. Reporter activity is restricted to the common ventricle and atria, but is absent from the aortic sac/truncus arteriosus and sinus venosus.
FIGURE 4.
Expression of the 184Ncx1-β-gal transgene in young adult hearts. (A) Transverse histological section of the left ventricular free wall showing mosaic LacZ staining in cardiomyocytes. (B) Whole mount LacZ staining of a central region of a heart showing LacZ-positive cells in and around a papillary muscle as well as the right ventricular free wall (arrowheads).
Although the minimal promotor is sufficient to direct the cardiomyocyte-restricted expression at the proper time in development, it is not sufficient to mediate upregulation in response to pressure overload.37 Clearly, these data indicate that although elements within the proximal 184 bases may be required for its expression there are additional distal elements necessary for Ncx1 regulation. We are currently analyzing the role of distal elements to determine which are required for upregulation and to maintain levels of expression in all cardiocytes. In spite of the loss of regulation by pressure overload and persistence of ubiquitous cardiocyte expression in the adult, it is remarkable that such a small region of the Ncx1 promotor is capable of driving the correct temporal- and tissue-specific expression.
REFERENCES
- 1.Kent RL, Rozich JD, McCollam PL, et al. Am. J. Physiol. 1993;265:H1024–H1029. doi: 10.1152/ajpheart.1993.265.3.H1024. [DOI] [PubMed] [Google Scholar]
- 2.Hasenfuss G, Reinecke H, Studer R, et al. Circ. Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 3.Pogwizd SM, Qi M, Yuan W, et al. Circ. Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 4.Hobai IA, O’Rourke B. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 5.Menick DR, Barnes KV, Thacker UF, et al. Ann. N. Y. Acad. Sci. 1996;779:489–501. doi: 10.1111/j.1749-6632.1996.tb44823.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng G, Hagen TP, Dawson ML, et al. J. Biol. Chem. 1999;274:12819–12826. doi: 10.1074/jbc.274.18.12819. [DOI] [PubMed] [Google Scholar]
- 7.Hasenfuss G, Meyer M, Schillinger W, et al. Basic. Res. Cardiol. 1997;92:87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- 8.Hasenfuss G, Schillinger W, Lehnart SE, et al. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 9.Shannon TR, Pogwizd SM, Bers DM. Circ. Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 10.Marx SO, Reiken S, Hisamatsu Y, et al. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 11.Pogwizd SM, Schlotthauer K, Li L, et al. Circ. Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 12.Weber CR, Piacentino V, III, Houser SR, Bers DM. Circulation. 2003;108:2224–2229. doi: 10.1161/01.CIR.0000095274.72486.94. [DOI] [PubMed] [Google Scholar]
- 13.Siegmund B, Koop A, Klietz T, et al. Am. J. Physiol. 1990;258:H285–H291. doi: 10.1152/ajpheart.1990.258.2.H285. [DOI] [PubMed] [Google Scholar]
- 14.Eigel BN, Hadley RW. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2184–H2190. doi: 10.1152/ajpheart.2001.281.5.H2184. [DOI] [PubMed] [Google Scholar]
- 15.Schafer C, Ladilov Y, Inserte J, et al. Cardiovasc. Res. 2001;51:241–250. doi: 10.1016/s0008-6363(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka M, Takano H, Suzuki M, et al. Biochem. Biophys. Res. Commun. 2004;314:849–853. doi: 10.1016/j.bbrc.2003.12.165. [DOI] [PubMed] [Google Scholar]
- 17.Muller JG, Isomatsu Y, Koushik SV, et al. Circ. Res. 2002;90:158–164. doi: 10.1161/hh0202.103231. [DOI] [PubMed] [Google Scholar]
- 18.Quednau BD, Nicoll DA, Philipson KD. Am. J. Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- 19.Barnes KV, Cheng G, Dawson MM, Menick DR. J. Biol. Chem. 1997;272:11510–11517. [PubMed] [Google Scholar]
- 20.Koushik SV, Bundy J, Conway SJ. Mech. Dev. 1999;88:119–122. doi: 10.1016/s0925-4773(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 21.Koban MU, Brugh SA, Riordon DR, et al. Mech. Dev. 2001;109:267–279. doi: 10.1016/s0925-4773(01)00548-2. [DOI] [PubMed] [Google Scholar]
- 22.Zechner D, Thuerauf DJ, Hanford DS, et al. J. Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Huang S, Sah VP, et al. J. Biol. Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 24.Zechner D, Craig R, Hanford DS, et al. J. Biol. Chem. 1998;273:8232–8239. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]
- 25.Nemoto S, Sheng Z, Lin A. Mol. Cell Biol. 1998;18:3518–3526. doi: 10.1128/mcb.18.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang F, Gardner DG. J. Clin. Invest. 1999;104:1603–1612. doi: 10.1172/JCI7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang F, Lu S, Gardner DG. Hypertension. 2000;35:188–192. doi: 10.1161/01.hyp.35.1.188. [DOI] [PubMed] [Google Scholar]
- 28.Liao P, Georgakopoulos D, Kovacs A, et al. Proc. Natl. Acad. Sci. USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews C, Ho PD, Dillmann WH, et al. Cardiovasc. Res. 2003;59:46–56. doi: 10.1016/s0008-6363(03)00329-8. [DOI] [PubMed] [Google Scholar]
- 30.Menick DR, Xu L, Kappler C, et al. Ann. N.Y. Acad. Sci. 2002;976:237–247. doi: 10.1111/j.1749-6632.2002.tb04746.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Muller JG, Withers PR, et al. Ann. N. Y. Acad. Sci. 2002;976:285–287. doi: 10.1111/j.1749-6632.2002.tb04751.x. [DOI] [PubMed] [Google Scholar]
- 32.Xu L, Kappler CS, Menick DR. J. Mol. Cell Cardiol. 2005;38:735–743. doi: 10.1016/j.yjmcc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Lemke LE, Bloem LJ, Fouts R, et al. J. Mol. Cell Cardiol. 2001;33:1527–1540. doi: 10.1006/jmcc.2001.1415. [DOI] [PubMed] [Google Scholar]
- 34.Rakhit RD, Kabir AN, Mockridge JW, et al. Biochem. Biophys. Res. Commun. 2001;286:995–1002. doi: 10.1006/bbrc.2001.5477. [DOI] [PubMed] [Google Scholar]
- 35.Kraev A, Chumakov I, Carafoli E. Genomics. 1996;37:105–112. doi: 10.1006/geno.1996.0526. [DOI] [PubMed] [Google Scholar]
- 36.Scheller T, Kraev A, Skinner S, Carafoli E. J. Biol. Chem. 1998;273:7643–7649. doi: 10.1074/jbc.273.13.7643. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Renaud L, Muller JG, et al. J. Biol. Chem. 2006;281:34430–34440. doi: 10.1074/jbc.M607446200. [DOI] [PMC free article] [PubMed] [Google Scholar]