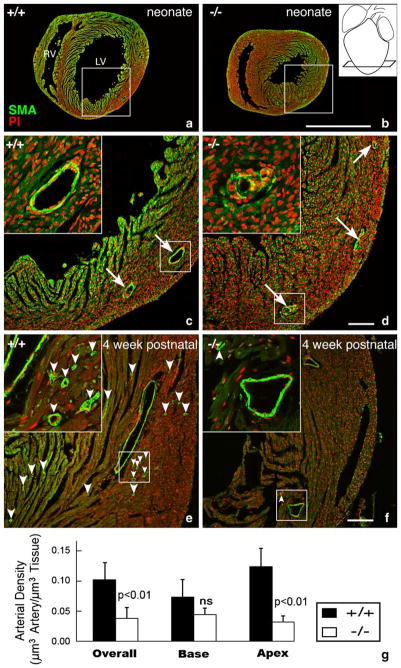
Fig. 6.
Apical disruption of coronary artery structure in the HF-1b knockout. (a–d) Alpha-smooth muscle actin immunolabeling (green) with propidium iodide nuclear counterstaining (red) highlight coronary arterial distributions in apical regions of a neonatal HF-1b+/+ (a, c) and HF-1b+/+ (b, d) ventricles. Inset in (b) shows section level at the apex. Boxed areas from (a) and (b) are shown at higher magnification in (c) and (d), respectively. Although the distribution of the main branches is preserved, vessel walls show discontinuous smooth muscle in the knockout at high magnification (insets). In the 4-week-old, apical sections stained with anti-smooth muscle actin and propidium iodide in (e) and (f). Although the main branches appear normally deployed in the HF-1b−/− heart, there is a paucity of small caliber vessels (best seen in the higher magnification insets) compared to that of the wildtype. Small arrowheads point to these smaller arterial profiles in (e) and (f). Scales=1 mm (a,b) and 100 μm (c, d, e, f). (g) Stereological analyses indicate a reduction in the total volume density of coronary arteries in the HF-1b−/− ventricle, as compared to HF-1b+/+, **(p <0.01). Furthermore, this decrease is seen in the apical half of the ventricle (p <0.01), but not in the base.