Abstract
Objective
To analyze the spectrum of neurological manifestations in children hospitalized with pandemic influenza A H1N1 virus of 2009 (pH1N1).
Design
Retrospective case series of children hospitalized from May 1, 2009, through November 30, 2009.
Setting
Tertiary-care children’s hospital in Colorado.
Patients
All hospitalized patients with pH1N1 with neurological consult or diagnosis, lumbar puncture, electroencephalogram, or neuroimaging were selected as suspected cases. These were systematically reviewed and selected for final analysis if confirmed by pre-established definitions as a neurological complication.
Results
Of 307 children with pH1N1, 59 were selected as having suspected cases of neurological complications. Twenty-three children were confirmed to have a neurological complication. Of these 23, 15 (65%) required intensive care monitoring. The median length of stay was 4 days. Seventeen (74%) had a preexisting neurological diagnosis. The most common manifestation was seizure with underlying neurological disease (in 62% of cases) followed by encephalopathy with or without neuroimaging changes (in 26% of cases). Results from a lumbar puncture showed elevated protein levels in 3 of 6 patients but no significant pleocytosis. Seven of the 9 electroencephalograms showed diffuse slowing, and findings from magnetic resonance imaging were abnormal in 5 of 6 children. Deaths occurred in 13% of patients, and short-term disability in 22%.
Conclusions
Children infected with pH1N1 presented with a wide spectrum of neurological manifestations, which occurred primarily in individuals with preexisting neurological conditions. These individuals had a severe disease course, evidenced by need for intensive care services and relatively high rates of mortality or neurological disability. Children with underlying neurological conditions should be particularly targeted for influenza prevention and aggressive supportive treatment at the onset of influenzalike symptoms.
Influenza is well known to have neurological manifestations and complications, including febrile and afebrile seizures, encephalitis, and acute necrotizing encephalopathy (ANE).1–4 Information on the clinical spectrum of illness in children with 2009 pandemic influenza A H1N1 (pH1N1) infection in the United States is still emerging. To better understand the neurological manifestations of pH1N1 in pediatric patients, we examined the clinical presentations, laboratory and neuroimaging findings, and outcomes in hospitalized children with pH1N1 infection who presented with neurological symptoms at The Children’s Hospital (TCH), Aurora, Colorado.
METHODS
We conducted a retrospective medical chart review of patients who were hospitalized at TCH, an academic, tertiary care, 314-bed hospital. All hospitalized children who had a respiratory specimen that was positive for the pH1N1 virus from May 1, 2009, through November 30, 2009, were entered into a database. These dates encompass both waves of the pH1N1 epidemic in our region. Cases with neurological consultation, diagnosis of altered mental status (AMS), seizure, encephalopathy, or encephalitis, or those who had a lumbar puncture (LP), electroencephalogram (EEG), magnetic resonance imaging (MRI) brain scan, MRI spine scan, or head computed tomographic (CT) scan were selected as suspected cases for medical chart review. Pandemic H1N1 infection was diagnosed by immunoassay (BinaxNOW Influenza A and B kits; Binax Inc, Scarborough, Maine) and by a multiplex polymerase chain reaction (PCR) platform using the xTag Respiratory Virus Panel system (Luminex Molecular Diagnostics, Toronto, Ontario, Canada).5 A previously published report,5 in addition to our clinical laboratory’s experience, has shown that specimens identified as nontypeable influenza A using this assay were uniformly confirmed to be the pH1N1 strain using the published Centers for Disease Control and Prevention assay.
All suspected cases were independently reviewed by 2 pediatric neurologists (S.K. and B.S.) for neurological complications using preestablished definitions (Table 1). In instances of disagreement, a third neurologist (J.P.) blindly reviewed the case, and the final designation was assigned based on agreement between 2 of the 3 neurologists. Cases concluded to have no neurological complication were excluded.
Table 1.
Definitions of Neurological Complications
| Designation | Neurological Complication and Designationa |
Occurrence No. (%) |
|---|---|---|
| 1 | Seizure without underlying neurological disorder or brain disease |
|
| A. Febrile: nonfocal seizure occurring in a child aged 6 mo to 6 y in association with fever (t > 38°C) |
0 | |
| B. Seizure with fever: seizure that is focal or patient age <6 mo or >6y old |
1 (4) | |
| C. Afebrile: focal or nonfocal seizure in any age in absence of fever |
0 | |
| 2 | Seizure with underlying neurological disorder or brain disease |
|
| A. Febrile: nonfocal seizure occurring in a child aged 6 mo to 6 y in association with fever (t > 38°C) |
5 (18) | |
| B. Seizure with fever: seizure that is focal or patient age <6 mo or >6y |
12 (44) | |
| C. Afebrile: focal or nonfocal seizure in any age in absence of fever |
0 | |
| 3 | Meningitis: clinical signs of meningitis, HA, photophobia, nuchal rigidity, AMS, and LP with pleocytosis |
0 |
| 4 | Encephalopathy: AMS for >24 h, confusion, lethargy, obtundation |
4 (15) |
| 5 | Encephalopathy with radiological changes |
3 (11) |
| 6 | Other | |
| A. Myelitis | 0 | |
| B. Stroke/vasculitis | 1 (4) | |
| 7 | Secondary | |
| A. Cardiac arrest leading to anoxic injury |
0 | |
| B. Respiratory arrest leading to anoxic injury |
0 | |
| C. Hypotension leading to cerebral infarction |
1 (4) |
Abbreviations: AMS, altered mental status; HA, headache; LP, lumbar puncture.
Children without neurological complication (eg, neonates with fever who underwent LP to rule out sepsis and were influenza positive) were excluded from analysis. In addition, 1 child with concurrent streptococcal pneumoniae meningoencephalitis was excluded. There were 27 neurological complications in 23 patients.
Any EEGs completed during the acute illness were independently visualized and reviewed by 2 neurologists (T.S. and A.R.B.-K.). If multiple EEGs or prolonged monitoring occurred, only the first EEG or initial 24 hours of the recording were assessed. The EEGs were evaluated for background, focality, slowing, epileptiform discharges, and electrographic seizures. Any new changes were noted when baseline comparison was available.
Neurological outcome was defined at the time of discharge based on medical and rehabilitation discharge summary and progress notes. Patients with no new disability were classified as having no disability; mild disability was defined as walking with no assistive device, mild modification of swallowing, or independent activities of daily living (ADLs) but not functioning at baseline; moderate disability was defined as being able to walk with an assistive device, moderate modification of swallowing, or some assistance with ADLs; and severe disability was defined as being unable to walk, unable to swallow, or requiring full assistance with ADLs. If patients had a neurological outcome of mild to severe at discharge, data from the last available follow-up visits were reviewed for any improvement.
The protocol and standardized data collection form were approved by the Colorado multiple institutional review board. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado.6 All statistical analysis was performed using SAS software, version, 9.2 (SAS Inc, Cary, North Carolina). Wilcoxon rank-sum test was used for continuous variables, and Fisher exact test was used for dichotomous variables.
RESULTS
From May 1 to November 30, 2009, 307 children with pH1N1 infection were hospitalized, of whom 59 were identified as having suspected neurological complications. Twenty-three (7.5% of the 307 hospitalized children with pH1N1) were determined to have a neurological complication (Table 1 and Table 2). Thirty-six cases did not meet criteria for any neurological complication. Four of the children had 2 distinct neurological complications.
Table 2.
Individual Case Description of All 23 Patients With Neurological Complications
| Patient No./Sex/Age, y | Neurological Complication |
H/O Epilepsy | H/O Neurologic Dx |
Received Tamiflu |
LOS, d | ICU | CSF WBC, cells/µL |
CSF RBC, cells/µL |
|---|---|---|---|---|---|---|---|---|
| 1a/F/4.9 | 5 | N | N | Y | 50 | Y | 9b | 80 |
| 2a/M/15.6 | 5 | N | N | Y | 7 | N | 2b | 114 |
| 3a/M/4.5 | 5, 2a | N | Infxn | Y | 28 | Y | 0 | 3 |
| 4/F/2.1 | 1b | N | N | Y | 1 | N | 1 | 4 |
| 5/F/3.2 | 2a | Y | DD | Y | 30 | Y | ||
| 6/M/0.9 | 2b | N | Onc | Y | 5 | Y | ||
| 7/M/4.3 | 2b | Y | SZ | Y | 3 | Y | ||
| 8/M/5.3 | 2b | Y | DD | Y | 2 | Y | ||
| 9/F/6.1 | 2b | N | PCH | Y | 1 | N | ||
| 10/M/6.4 | 2b | Y | DD | Y | 4 | Y | 3 | 18 |
| 11/F/7.6 | 2b | Y | SCN1a,c | Y | 2 | Y | ||
| 12/F/9.7 | 2b | Y | SZ | Y | 6 | N | ||
| 13/F/10.2 | 2b | N | SOD | Y | 2 | N | ||
| 14/M/11.7 | 2b | Y | DD | Y | 2 | N | ||
| 15/M/13.2 | 2b | Y | DD | Y | 3 | Y | ||
| 16/M/14.5 | 2b | Y | ACCc | Y | 4 | Y | ||
| 17/F/15.5 | 2b | N | Stroke | N | 4 | Y | ||
| 18/M/12.0 | 4 | N | N | Y | 2 | Y | 1 | 1 |
| 19/F/0.7 | 4, 2a | Y | ACC | Y | 9 | N | 4 | 2 |
| 20/F/3.5 | 4, 2a | Y | DD/CP | N | 6 | Y | ||
| 21/M/19.0 | 4, 2a | Y | SCN1a, c | Y | 1 | N | ||
| 22/M/5.9 | 6b | N | N | Y | 27 | Y | ||
| 23/M/11.3 | 7c | N | N | Y | 43 | Y |
| Patient No./Sex/ Agey |
CSF Glucose Level, mg/dL |
CSF Protein Level, g/dL |
EEG | CTH | MRI | Disability at Discharge |
Disability (mo After Discharge) |
|---|---|---|---|---|---|---|---|
| 1a/F/4.9 | 54 | 0.069 | Diffuse slowing | Hypodensities in brainstem | Abnl | Mod | Mild (2.8) |
| 2a/M/15.6 | 56 | 0.040 | NL | NL | Abnl | None | |
| 3a/M/4.5 | 134 | 0.290 | Diffuse slowing | Cerebral edema | Abnl | Fatal | |
| 4/F/2.1 | 79 | 0.012 | ND | ND | ND | None | |
| 5/F/3.2 | Diffuse slowing, multifocal ED | ND | ND | Mild | Mild (1.6) | ||
| 6/M/0.9 | Diffuse slowing, multifocal ED | ND | Baseline | None | |||
| 7/M/4.3 | ND | ND | ND | None | |||
| 8/M/5.3 | ND | ND | ND | None | |||
| 9/F/6.1 | Multifocal ED | ND | ND | None | |||
| 10/M/6.4 | 76 | 0.020 | Diffuse slowing, multifocal ED | ND | ND | Mild | None (2.4) |
| 11/F/7.6 | ND | NL | ND | None | |||
| 12/F/9.7 | ND | ND | ND | None | |||
| 13/F/10.2 | ND | NL | ND | None | |||
| 14/M/11.7 | ND | ND | ND | None | |||
| 15/M/13.2 | ND | Baseline | ND | None | |||
| 16/M/14.5 | ND | ND | ND | None | |||
| 17/F/15.5 | ND | Baseline | ND | None | |||
| 18/M/12.0 | 69 | 0.019 | ND | NL | ND | None | |
| 19/F/0.7 | 73 | 0.076 | ND | ND | ND | Fatal | |
| 20/F/3.5 | Diffuse slowing | NL | ND | None | |||
| 21/M/19.0 | ND | ND | ND | Fatal | |||
| 22/M/5.9 | ND | Multifocal cortical hypodensities | Abnl | Mild | None (3) | ||
| 23/M/11.3 | Diffuse slowing, electrographic seizure |
Focal gray white junction, hypodensity | Abnl | Mild | Mild (9.5) |
Abbreviations: Abnl, abnormal; ACC, agenesis of corpus callosum; CP, cerebral palsy; CSF, cerebrospinal fluid; CTH, computed tomography of the head; DD, developmental delay; Dx, diagnosis; ED, epileptiform discharge; H/O, history of; ICU, intensive care unit; Infxn, previous encephalitis; LOS, length of stay; N, No; ND, not done; NL, normal; Onc, oncology [brain tumor]; PCH, pontocerebellar hypoplasia; RBC, CSF red blood cell count; SCN1a, Dravets; SOD, septic optic dysplasia; SZ, epilepsy only; WBC, white blood cell count; Y, yes.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555. To convert protein to grams per liter, multiply by 10.0.
These 3 cases are detailed in the “Case Examples” subsection of the “Methods” section.
The CSF polymerase change reaction samples were negative for pandemic influenza A H1N1.
Cases with intractable seizures.
The median age of pH1N1-positive hospitalized patients with neurological complications was 6.4 years, and 57% were male. More than half (65%) required intensive care unit (ICU) support, and the median length of hospital stay (LOS) was 4 days. In contrast, in the nonneurological cohort (n = 284), 65 patients (24%) required ICU support (P<.001) and the median LOS was 3 days (P=.10). Twenty-one of the 23 patients (91%) had fever, 17 (74%) had cough, 13 (57%) were hypoxic, and 21 (91%) were treated with oseltamivir. Patients had onset of neurological symptoms a median of 1 day after the onset of their illness and fever onset. Eleven patients (48%) were admitted to the hospital concurrent with the onset of their neurological symptoms (Table 3).
Table 3.
Characteristics of 23 Hospitalized Patients With pH1N1 Who Had a Neurological Complication
| Characteristic | Value | Range |
|---|---|---|
| Age, median (IQR), y | 6.4 (4.3–12) | 0.7–19 |
| Male, No. (%) | 13 (57) | |
| Admitted to ICU, No. (%) | 15 (65) | |
| Length of stay, median (IQR), d | 4 (2–25) | 1–52 |
| Time, median (IQR), d | ||
| From illness onset to neurological symptoms onset |
1 (0–4) | 0–9 |
| From fever onset to neurological symptoms onset |
1 (0–3.5) | −1 to 9 da |
| From neurological symptoms onset to admission |
0 (0–2) | −2 to 7 db |
| Neurological symptoms at illness onset, No. (%) |
9 (39) | |
| Neurological symptoms at fever onset, No. (%) |
7 (30) | |
| Neurological symptoms at time of admission, No. (%) |
11 (48) |
Abbreviations: ICU, intensive care unit; IQR interquartile range; pH1N1, pandemic influenza A H1N1.
A negative number refers to neurological symptoms starting before the onset of fever.
A negative number refers to neurological symptoms presenting after admission to hospital.
Seventeen of the 23 cases (74%) had an underlying neurological diagnosis, 12 (52%) of whom had a diagnosis of epilepsy. Three of the 12 had intractable seizures. Seizure with fever in children with underlying neurological disease (17 cases [63%]) was the most common neurological manifestation, and encephalopathy (7 cases [26%]) was the second most common. Of the children presenting with seizures, only 1 (6%) had no underlying neurological disorder, and 12 (75%) had a previous history of seizures. Of the 7 children presenting with encephalopathy, 3 (43%) were previously healthy, 3 (43%) had an underlying seizure disorder, and 1 (14%) had an underlying neurological condition without seizures.
CEREBROSPINAL FLUID ANALYSIS
Lumbar punctures were performed in 7 of the 24 individuals. All cerebrospinal fluid (CSF) bacterial cultures were negative for bacteria. Two CSF samples had influenza PCR analysis, both of which were negative for the virus (cases 1 and 2). Only 1 patient had a significant pleocytosis (white blood cell count > 5 cells/µL) with 9 WBCs (44% neutrophils, 45% lymphocytes). The median protein level was 0.04 g/dL with 3 of the 7 children having clinically significant protein level elevation (range, 0.07–0.29 g/dL). Two of the 3 children with elevated protein levels died (including case 3), and the third had clinically significant neurological disability at discharge (case 1). (To convert protein to grams per liter, multiply by 10.0.)
NEUROIMAGING
Eleven of the 23 patients (48%) had head CTs without contrast, 1 of whom had multiple studies. Results of 3 of the 11 patients’ CT scans (27%) were abnormal (Table 2). Six children had brain MRIs, the results of 5 of which were abnormal (Table 4).
Table 4.
Summary of MRI Findings of the 6 Hospitalized Children With pH1N1 Who Had MRI Scans
| Patient No. |
Days From Illness Onset |
Abnl Result |
Finding | Enhancement | Comments/Other |
|---|---|---|---|---|---|
| 1 | 5 | Yes | T2 lesions of pons, midbrain, thalami, medial temporal lobes, and external capsules bilaterally |
None | Results from MRA and MRV head and neck negative/MRI spine negative |
| 2 | 7 | Yes | 2 Symmetric foci of high T2 signal in the occipital horns and trigones of both lateral ventricles |
None | Repeated 4 d later with no change |
| 3 | 20 | Yes | T2 and restricted diffusion symmetrically in the deep gray structures, BG, thalami, pons; diffuse bilateral cerebral cortical and subcortical volume loss and abnormal central and periventricular white matter signal |
Yes, present at site of ICP bolt |
|
| 6 | 1 | No | Status post–glioblastoma multiform resection with focal encephalomalacia, stable compared with previous results |
Yes, stable from previous |
|
| 23 | 6 | Yes | Infarcts involving the right frontal and right occipital region | None | MRA: diminished caliber of the right ICA and MCA |
| 24 | 5 | Yes | Patchy symmetric restricted diffusion bilaterally predominately in subcortical white matter. |
None | MRA: all major vessels of the circle of Willis diffusely small and ACA, MCAs small |
Abbreviations: Abnl, Abnormal; ACA, anterior cerebral artery; ICA, internal carotid artery; ICP, intracranial pressure; MCA; middle cerebral artery; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; pH1N1, pandemic influenza A H1N1 virus.
ELECTROENCEPHALOGRAMS
A total of 9 patients (39%) had at least 1 EEG performed during their illness. Four of the 9 patients had previous EEGs for comparison. Seven of them presented with clinical seizures. Of the 9 patients with EEG studies, the result for 1 was normal, 1 had focal electrographic seizures recorded, 5 had multifocal epileptiform discharges, and 7 had diffuse slowing consistent with encephalopathy (Table 2).
NEUROLOGICAL OUTCOMES
Fifteen of the 23 patients (65%) had no neurological disability at discharge. Four patients (17%) had mild disability, and 1 (4%) had moderate disability. Three patients (13%) died compared with 6 of the hospitalized patients without neurological complications (2%) (P=.02). Of the 5 individuals with disability at discharge, 3 showed some improvement at follow-up (Table 2).
CASE EXAMPLES
Case 1
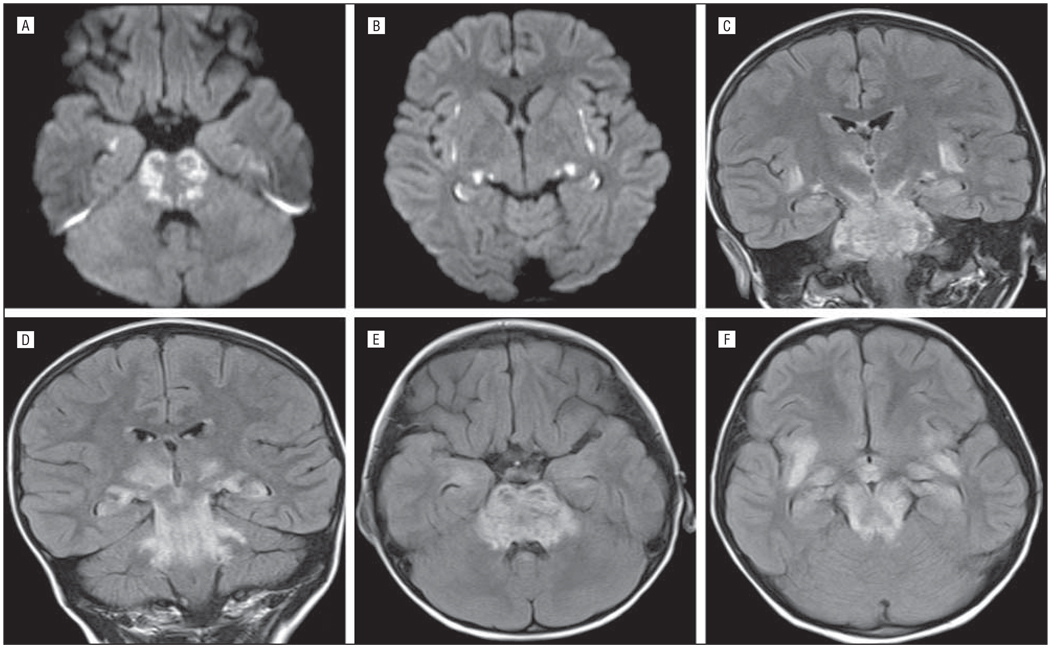
A healthy 4-year-old girl presented with a 1-day history of fever (102.5°F) and several hours of lethargy, dilated pupils, and unresponsiveness. She had no headache or respiratory or gastrointestinal tract symptoms. Results from an LP revealed 9 WBCs/µL (31% neutrophils, 63% lymphocytes); 80 red blood cells/µL (RBCs); a glucose level of 54 mg/dL; and protein level of 0.07 g/dL. (To convert glucose to millimoles per liter, multiply by 0.0555.) The EEG demonstrated focal right hemispheric suppression, and the MRI scan showed clinically significant abnormalities (Figure 1 and Table 4). Neurological examination showed a minimally responsive intubated child who was not following commands and without speech. Brainstem reflexes were intact. She had increased tone and reflexes in her extremities, with withdrawal to pain in all 4 extremities. She was treated initially with oseltamivir and then with peramivir. Because of concerns for possible acute disseminated encephalomyelitis (ADEM) vs ANE she was treated with methylprednisolone sodium succinate, 30 mg/kg/d for 3 doses, and intravenous immunoglobulin, 2 g/kg, over 2 days. She was hospitalized for 53 days, including 9 days in the ICU and 37 days of inpatient acute rehabilitation. She significantly improved throughout her hospitalization and was discharged with a moderate disability.
Figure 1.
Case 1. The patient had restricted diffusion in rostral pons (A), insular cortex (B), and medial temporal lobe (A and B). Fluid-attenuated inversion recovery shows extensive symmetric involvement of the brainstem, insular cortex, and thalami on coronal (C and D) and axial (E and F) sequences.
Case 2
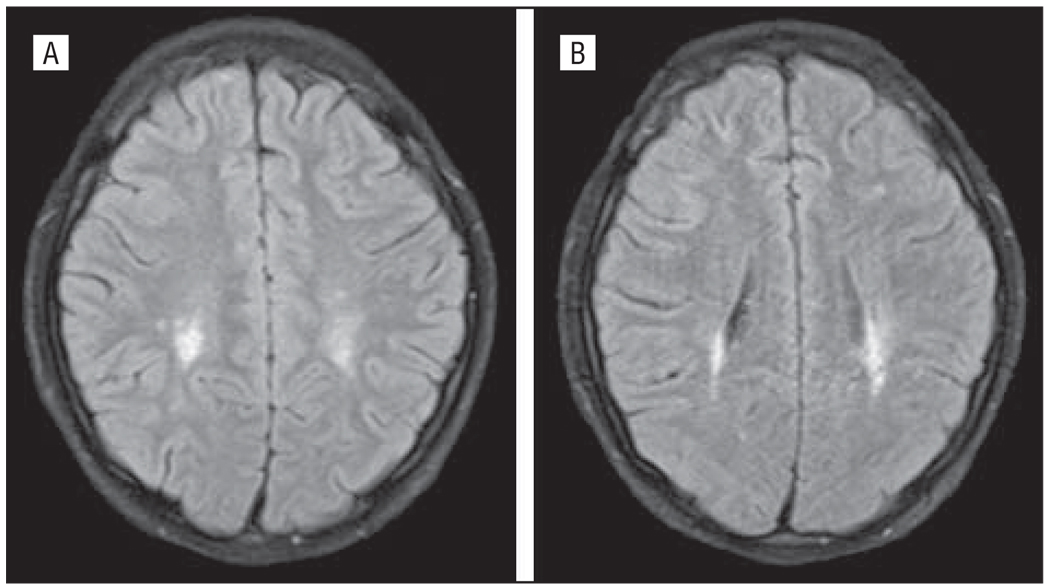
A healthy 15-year-old boy presented with a history of 6 days of AMS with waxing and waning alertness and arousal and 1 day of delusional symptoms. Symptoms included being withdrawn, reclusive, and sleepy. Formal neuro-psychological testing found him to be confused, easily disorganized, disinhibited, perseverative, with markedly abnormal frontal lobe functions. His family reported that he had experienced 7 days of dry cough but no fever or gastrointestinal tract symptoms. Findings from a comprehensive metabolic panel, standard and expanded urine toxicologic tests, complete blood cell count, and head CT imaging were normal. Results from an LP revealed 2 WBCs/µL, 114 RBCs/µL, a glucose level of 56 mg/dL, and a protein level of 0.04 g/dL. The CSF influenza PCR testing was negative for the virus. Brain MRI scans showed T2 signal lesions of uncertain significance on hospital day 2 (Figure 2), which were unchanged 4 days later. The patient was treated with oseltamivir and discharged after 9 days with no neurologic disability.
Figure 2.
Case 2. The patient had fluid-attenuated inversion recovery signal abnormality in posterior periventricular white matter signal symmetrically (A and B).
Case 3
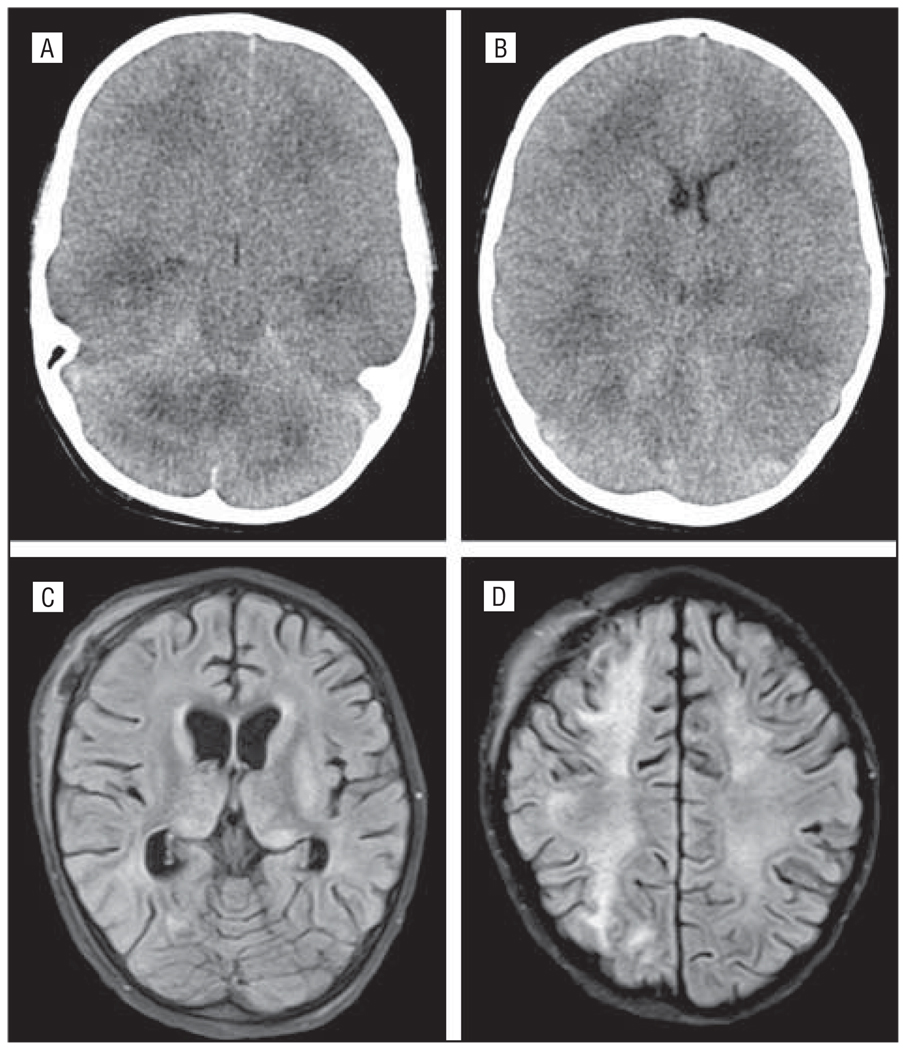
A 4-year-old boy with a history of benign congenital neutropenia and an episode of presumed viral meningitis at 1 year of age was well until 2 days prior to presentation when he developed generalized malaise. The following day he developed a fever of 104°F, nonproductive cough, and sleepiness. On the day of admission he developed rigors, agitation, generalized weakness, and a 10-minute generalized tonic seizure. At an outside hospital, he was treated with lorazepam and phenytoin for a second seizure and then with phenobarbital en route to TCH after a third seizure. Findings of the initial neurologic examination at TCH were notable for intact brain-stem reflexes, upgoing toes, hypotonia, brisk symmetric withdrawal of the upper extremities, and triple flexion withdrawal of the lower extremities. Continuous EEG demonstrated diffuse slowing with no seizures. The results of the LP showed no WBCs, 3 RBCs/µL, a protein level of 0.29 g/dL, and a glucose level of 134 mg/dL. The peripheral WBC count was initially low at 2.1 WBCs/µL with 14% neutrophils, 41% lymphocytes, and 13% mono cytes. Hemoglobin, serum aminotransferases, alkaline phosphatase, lactate dehydrogenase, and ammonia levels and platelet counts were within reference range. The following morning, his neurological status changed, necessitating intracranial bolt placement with initial pressure of 63 mm Hg. A head CT scan is shown in Figure 3. On hospital day 16 he developed cardiopulmonary arrest requiring chest compressions and treatment with epinephrine. An MRI scan performed on day 20 is shown in Figure 3. A brain biopsy specimen taken on day 22 showed no lymphocytic inflammation; however, it showed small vessel hyalinization, microglial activation, and reactive gliosis. Influenza PCR on a brain tissue specimen was negative for the virus. Extensive metabolic and genetic testing was nondiagnostic. After 28 days and no significant improvement, aggressive life support was discontinued, and the patient died. Autopsy findings showed severe multifocal bilateral necrotizing leukoencephalopathy in deep cerebral white matter, thalami, basal ganglia, limbic structures, and pons. In addition, there were remote areas of patchy pallor in the deep and frontal white matter thought to be due to his prior episode of encephalopathy. Cerebellar gyral atrophy with corresponding damage in olivary nuclei were thought to be due to previous hypoxic ischemic injury. The final pathological diagnosis was ANE with influenza (pH1N1).
Figure 3.
Case 3. The patient showed on computed tomographic images diffuse cerebral edema and basilar cistern effacement (A and B) on hospital day 2, and fluid-attenuated inversion recovery imaging on day 20 showed extensive signal changes in the thalami and diffuse white matter (C and D).
COMMENT
We report our experience of neurological complications seen in hospitalized children with pH1N1. We found that 7.5% of hospitalized children presented with neurological manifestations. To our knowledge, this is the largest and most comprehensive report of the incidence of neurological complications in hospitalized children infected with pH1N1. It is consistent with reports for pH1N17 and other strains of influenza viruses.1
Currently, there are a number of case reports and case series documenting the neurological manifestations and complications of pH1N1 infection in children.7–15 In a brief report,8 4 pediatric cases presenting with seizures and or encephalopathy were described. All 4 had minimal CSF pleocytosis, EEG abnormalities occurred in 3, and MRI abnormalities were found in 1 of the 3 patients who underwent imaging. In another case series, 3 children had AMS, EEG abnormalities consistent with acute encephalopathy, and lack of other organ dysfunction.11 Two of these 4 patients showed imaging abnormalities, 1 with thalamic swelling and diffusion-weighted changes, and the second with initial normal MRI results and volume loss on subsequent MRI scans. In 2 other case series including adults, the children similarly presented with seizures and AMS with no abnormalities on CSF analysis and neuro-imaging except for 1 child diagnosed as having brain atrophy.13,14 Other case reports and series have demonstrated children with pH1N1 presenting with complex febrile seizure,16 ANE,9,17 meningioencephalitis,7,10 neuro-psychiatric symptoms,12 encephalopathy,15 and ADEM.7,15
In our study, the most common neurological manifestation in hospitalized children with pH1N1 was seizures. All of these patients were febrile, and all but 1 had known underlying epilepsy. Febrile seizures are a common event for children with influenza and may result in hospitalization.18,19 We had a low incidence of simple febrile seizures, likely because children with simple febrile seizures are not routinely admitted to the hospital at our institution. Because of this, the overall incidence of central nervous system manifestations of pH1N1 influenza may be higher than 7.5%. There were no children hospitalized for seizures without a fever. It is not clear whether seizures in the pH1N1 population are a nonspecific effect of a viral infection, cytokine release, or fever by lowering seizure threshold, or a more specific neurotropic effect of pH1N1 virus.19–21
The second most common neurological manifestation was encephalopathy. Our findings support previous observations that encephalopathy in influenza has a wide spectrum of presentations and is associated with poor neurological outcomes. Several reports,22,23 most from Japan, have documented influenza-associated ANE, which was originally defined by Mizuguchi23 according to the following criteria: (1) acute encephalopathy following a viral febrile illness with rapid deterioration in the level of consciousness and convulsions; (2) lack of CSF pleocytosis, but increased CSF protein commonly observed; (3) CT or MRI evidence of symmetric, multifocal brain lesions involving the bilateral thalami; (4) elevation of serum aminotransferases with no increase in blood ammonia; and (5) exclusion of other resembling diseases. The pathogenesis of ANE has not been clearly defined but is thought to be related to increased cytokine and macrophage activation.22–25
Both cases 1 and 3 presented with an acute encephalopathy, fever, and seizures with bilateral thalamic T2 changes. By Mizuguchi’s23 original diagnostic criteria for ANE, case 1 would favor a diagnosis of ADEM over ANE based on the presence of a mild pleocytosis and absence of a transaminitis or thrombocytopenia. Symmetric basal ganglia and thalamic gray matter lesions have been commonly reported in ADEM and are among the 4 patterns used to describe MRI findings in ADEM.26,27 As seen in this patient, the onset of ADEM is acute and typically occurs 2 days to 1 month after infection or vaccination.26,27 Histologically, there is a paucity of inflammatory cells in ANE, whereas ADEM demonstrates perivenule macrophages and T cells associated with demyelination.27 Some studies have used the complete resolution of MRI brain changes (ie, cavitary lesions) or a biphasic course in which bilateral thalamic lesions were not found at relapse as evidence supporting an ADEM diagnosis.26 Results from histological and repeated MRI studies, however, were not available for case 1.
Case 3 exemplified a CSF profile more typical of ANE with a significantly elevated protein level and absence of WBCs. A similar, but less dramatic, CSF profile (1 WBC/ µL, 0 RBCs, normal glucose level, and protein level of 135 mg/dL) was present with this patient’s prior presentation of encephalopathy and seizures that was initially presumed to be viral encephalitis. During his prior episode, the results from his MRI scan were normal, his aspartate transaminase level was mildly elevated at 73 U/L (1.22 µkat/L), but he showed no other evidence of an insult characteristic of ANE or ADEM. The patient was not treated with steroids, and he clinically recovered with little impairment. In retrospect, however, based on autopsy findings, there was likely some white matter insult at that time. The patient’s second presentation demonstrated bilateral thalamic lesions on MRI. However, he did not show other abnormalities common to ANE, including throm-bocytopenia or transaminitis. Nevertheless, his 2 clinical presentations of encephalopathy with CSF albumino-cytologic dissociation in conjunction with a postmortem diagnosis of ANE may reflect the clinical spectrum of ANE or the possibility of a biphasic course.
Although most of our patients with encephalopathy presented with symptoms of somnolence, decreased level of consciousness, or loss of consciousness, 1 patient presented with abnormal behaviors and hallucinations (case 2). Fevers have been associated with similar mental status changes, but this patient was afebrile. Although unusual, acute psychosis was reported in 6 of 148 patients with influenza (4%) during the 2002 epidemic in Japan and was reported in 1 case report of a patient with pH1N1.12,21
Six of our 7 patients with LPs had no clinically significant pleocytosis. Interestingly, 3 children, all of whom had poor outcomes, presented with elevated CSF protein levels. Concordant with previous reports that influenza RNA is rarely present in CSF,7,28,29 results from influenza PCR on our 2 CSF samples were negative for influenza RNA. In a third patient, a brain biopsy specimen was negative for influenza. It continues to be unclear whether the CNS manifestations of influenza are from a direct effect of the virus or a secondary inflammatory effect.
Similar to other reports of influenza-related CNS illnesses,21,30 children with pH1N1 who had neurological manifestations had a severe disease course. This was evidenced by the fact that approximately two-thirds of our cohort required ICU care. In addition, this subset of patients had an overall mortality of 13% compared with 2.1% in all of the hospitalized children with pH1N1 at our institution (Bagdure et al31). Furthermore, 74% of the children who presented with a neurological manifestation had a preexisting neurological diagnosis. Of note, none of our patients had an underlying neuromuscular disorder, and all of our patients in our neuromuscular clinic who acquired pH1N1 influenza had a mild disease course (J.P., unpublished observation, 2010), suggesting that muscle weakness or dysfunction did not predispose to neurological complications or severe disease. This highlights what others have reported1: that children with underlying neurological disease have increased risk of a neurological complication and a more severe disease course from influenza.
Vaccines for pH1N1 were not available in our region during most of the pandemic in 2009; therefore, a majority of our patients were unvaccinated. It is possible that timely vaccination could have prevented some of these complications.
In conclusion, neurological manifestations were seen in approximately 7.5% of hospitalized patients with pH1N1 and primarily in patients with underlying neurological conditions. The most common neurological manifestations seen were seizures and encephalopathy. In general, these patients had a severe disease course requiring prolonged hospital stays and ICU support, with a high percentage of deaths and neurological disability. Children with underlying neurological conditions should be particularly targeted for influenza prevention and aggressive supportive treatment at the onset of influenzalike symptoms.
Acknowledgments
Funding/Support: The research for this study was supported by Colorado Clinical and Translational Sciences Institute grant 1UL1 RR025780.
Footnotes
Author Contributions: Dr Dominguez had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Kedia and Stroud contributed equally to this study. Study concept and design: Kedia, Stroud, Parsons, Brooks-Kayal, and Dominguez. Acquisition of data: Kedia, Stroud, Parsons, Schreiner, Curtis, Bagdure, Brooks-Kayal, Glode, and Dominguez. Analysis and interpretation of data: Kedia, Stroud, Parsons, Schreiner, Curtis, Brooks-Kayal, and Dominguez. Drafting of the manuscript: Kedia, Stroud, and Dominguez. Critical revision of the manuscript for important intellectual content: Kedia, Stroud, Parsons, Schreiner, Bagdure, Brooks-Kayal, Glode, and Dominguez. Statistical analysis: Dominguez. Obtained funding: Dominguez. Administrative, technical, and material support: Kedia and Dominguez. Study supervision: Parsons, Brooks-Kayal, Glode, and Dominguez.
Financial Disclosure: None reported.
Additional Contributions: Greta Wilkening, PsyD (The Children’s Hospital), provided support and neuropsychiatric testing; Bette Kleinschmidt-DeMasters, MD (University of Colorado School of Medicine), provided expertise and review of pathological specimens; and Kenneth L. Tyler, MD (University of Colorado School of Medicine), provided critical evaluation of the manuscript.
REFERENCES
- 1.Newland JG, Laurich VM, Rosenquist AW, et al. Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr. 2007;150(3):306–310. doi: 10.1016/j.jpeds.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 2.Studahl M. Influenza virus and CNS manifestations. J Clin Virol. 2003;28(3):225–232. doi: 10.1016/s1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114–124. doi: 10.1016/j.tmaid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Weitkamp JH, Spring MD, Brogan T, Moses H, Bloch KC, Wright PF. Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr Infect Dis J. 2004;23(3):259–263. doi: 10.1097/01.inf.0000115631.99896.41. [DOI] [PubMed] [Google Scholar]
- 5.Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45(3):191–195. doi: 10.1016/j.jcv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calitri C, Gabiano C, Garazzino S, et al. Clinical features of hospitalised children with 2009 H1N1 influenza virus infection. Eur J Pediatr. 2010;169(12):1511–1515. doi: 10.1007/s00431-010-1255-y. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Neurologic complications associated with novel influenza A (H1N1) virus infection in children, Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58(28):773–778. [PubMed] [Google Scholar]
- 9.Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol. 2010;40(2):200–205. doi: 10.1007/s00247-009-1487-z. [DOI] [PubMed] [Google Scholar]
- 10.Haktanir A. MR imaging in novel influenza A(H1N1)-associated meningoencephalitis. AJNR Am J Neuroradiol. 2010;31(3):394–395. doi: 10.3174/ajnr.A2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baltagi SA, Shoykhet M, Felmet K, Kochanek PM, Bell MJ. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med. 2010;11(2):179–184. doi: 10.1097/PCC.0b013e3181cf4652. [DOI] [PubMed] [Google Scholar]
- 12.German-Diaz M, Pavo-Garcia R, Diaz-Diaz J, Giangaspro-Corradi E, Negreira-Cepeda S. Adolescent with neuropsychiatric symptoms associated with novel influenza A (H1N1) virus infection. Pediatr Infect Dis J. 2010;29(6):570–571. doi: 10.1097/INF.0b013e3181d411a9. [DOI] [PubMed] [Google Scholar]
- 13.Noriega LM, Verdugo RJ, Araos R, et al. Pandemic influenza A (H1N1) 2009 with neurological manifestations, a case series. Influenza Other Respi Viruses. 2010;4(3):117–120. doi: 10.1111/j.1750-2659.2010.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan K, Prerna A, Leo YS. Surveillance of H1N1-related neurological complications. Lancet Neurol. 2010;9(2):142–143. doi: 10.1016/S1474-4422(10)70015-6. [DOI] [PubMed] [Google Scholar]
- 15.Webster RI, Hazelton B, Suleiman J, Macartney K, Kesson A, Dale RC. Severe encephalopathy with swine origin influenza A H1N1 infection in childhood: case reports. Neurology. 2010;74(13):1077–1078. doi: 10.1212/WNL.0b013e3181d6b113. [DOI] [PubMed] [Google Scholar]
- 16.O’Leary MF, Chappell JD, Stratton CW, Cronin RM, Taylor MB, Tang YW. Complex febrile seizures followed by complete recovery in an infant with high-titer 2009 pandemic influenza A (H1N1) virus infection. J Clin Microbiol. 2010;48(10):3803–3805. doi: 10.1128/JCM.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariotti P, Iorio R, Frisullo G, et al. Acute necrotizing encephalopathy during novel influenza A (H1N1) virus infection. Ann Neurol. 2010;68(1):111–114. doi: 10.1002/ana.21996. [DOI] [PubMed] [Google Scholar]
- 18.Chiu SS, Tse CY, Lau YL, Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108(4):E63. doi: 10.1542/peds.108.4.e63. [DOI] [PubMed] [Google Scholar]
- 19.Kwong KL, Lam SY, Que TL, Wong SN. Influenza A and febrile seizures in childhood. Pediatr Neurol. 2006;35(6):395–399. doi: 10.1016/j.pediatrneurol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Kawada J, Kimura H, Ito Y, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infect Dis. 2003;188(5):690–698. doi: 10.1086/377101. [DOI] [PubMed] [Google Scholar]
- 21.Morishima T, Togashi T, Yokota S, et al. Collaborative Study Group on Influenza-Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35(5):512–517. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- 22.Mastroyianni SD, Gionnis D, Voudris K, Skardoutsou A, Mizuguchi M. Acute necrotizing encephalopathy of childhood in non-Asian patients: report of three cases and literature review. J Child Neurol. 2006;21(10):872–879. doi: 10.1177/08830738060210101401. [DOI] [PubMed] [Google Scholar]
- 23.Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19(2):81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 24.Akiyoshi K, Hamada Y, Yamada H, Kojo M, Izumi T. Acute necrotizing encephalopathy associated with hemophagocytic syndrome. Pediatr Neurol. 2006;34(4):315–318. doi: 10.1016/j.pediatrneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Okumura A, Mizuguchi M, Kidokoro H, et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009;31(3):221–227. doi: 10.1016/j.braindev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 27.Tenembaum S, Chitnis T, Ness J, Hahn JS. International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68(16) suppl 2:S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 28.Steininger C, Popow-Kraupp T, Laferl H, et al. Acute encephalopathy associated with influenza A virus infection. Clin Infect Dis. 2003;36(5):567–574. doi: 10.1086/367623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito Y, Ichiyama T, Kimura H, et al. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58(4):420–425. doi: 10.1002/(sici)1096-9071(199908)58:4<420::aid-jmv16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Kasai T, Togashi T, Morishima T. Encephalopathy associated with influenza epidemics. Lancet. 2000;355(9214):1558–1559. doi: 10.1016/S0140-6736(05)74614-6. [DOI] [PubMed] [Google Scholar]
- 31.Bagdure D, Curtis DJ, Dobyns E, Glode MP, Dominguez SR. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PLoS ONE. doi: 10.1371/journal.pone.0015173. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]