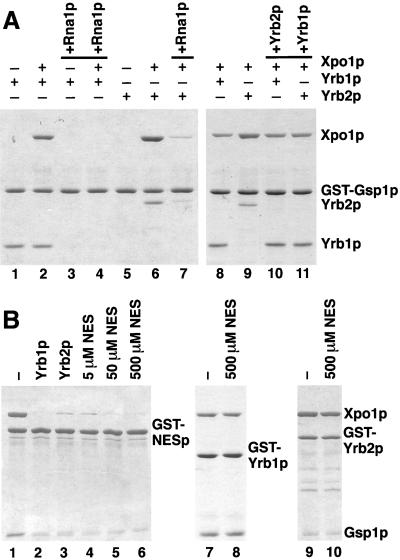
Figure 4.
The association of Yrb1p and Yrb2p with Xpo1p/Gsp1p-GTP is sensitive to Rna1p but resistant to excess amounts of NES peptides. (A) GST-Gsp1p-GTP (4 μg per reaction) was immobilized on glutathione Sepharose and incubated for 30 min at 4°C with 5 μg of Yrb1p, 5 μg of Yrb2p, and/or 12 μg of Xpo1p, as indicated. Bound material was washed three times and either eluted with SDS sample buffer (lanes 1, 2, 5, 6, 8, and 9) or incubated further for 15 min at 4°C with 1 μg of Rna1p (lanes 3, 4, and 7), 10 μg of Yrb2p (lane 10), or 10 μg of Yrb1p (lane 11), and then washed again three times. All samples were analyzed by SDS PAGE and Coomassie blue staining. (B) GST fusions (6 μg per reaction) to PKI-NES-2GFP (GST-NESp)(lanes 1–6), Yrb1p (lanes 7 and 8), or Yrb2p (lanes 9 and 10) were immobilized on glutathione Sepharose and incubated for 30 min at 4°C with 10 μg of Gsp1p-GTP and 12 μg of Xpo1p. After three washes, the reactions were further incubated for 15 min at 4°C with buffer alone (lanes 1, 7, and 9), with 6 μg of Yrb1p (lane 2), with 6 μg of Yrb2p (lane 3), or with the indicated amounts of peptides corresponding to the PKI-NES (lanes 4–6, 8, and 10). After three washes, bound material was analyzed by SDS PAGE and Coomassie blue staining.