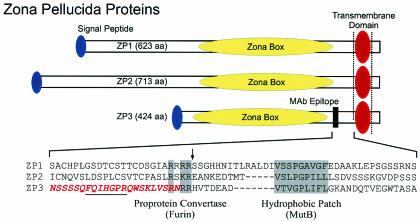
FIG. 7.
Zona pellucida proteins. Mouse ZP1 (623 amino acids), ZP2 (713 amino acids), and ZP3 (424 amino acids) each has a signal peptide (blue oval) to direct it into a secretory pathway, an ∼260-amino-acid zona domain that contains eight conserved cysteine residues (yellow oval) and a transmembrane domain (red oval) near the carboxyl termini followed by a short cytoplasmic tail. The amino acid sequences of the three mouse zona proteins are aligned between a ZP3-specific monoclonal antibody binding site (darkened box, amino acids 336 to 342) and the ZP3 transmembrane domain (amino acids 387 to 410). Each zona protein has a conserved cleavage site (arrow) for proprotein convertase/furin (R-X-R/K-R) and a hydrophobic patch that are 36 to 53 and 25 to 40 amino acids, respectively, N terminal to the transmembrane domain. The carboxyl terminus of ZP3 defined by MS is shown in red letters. The binding site of the monoclonal antibody that recognizes ZP3 in the extracellular zona pellucida is underlined.