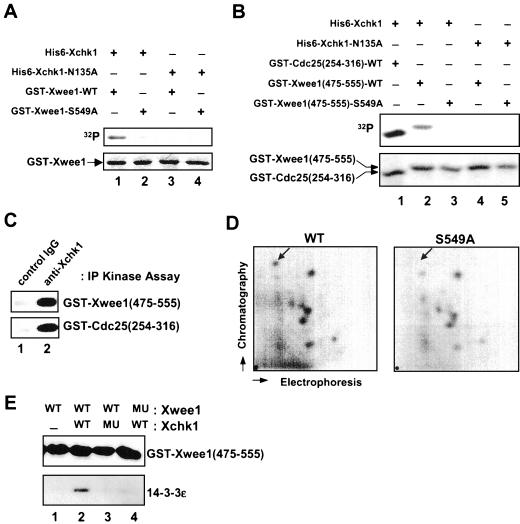
Figure 2.
Phosphorylation of Ser-549 on Xwee1 by Xchk1 mediates the binding of 14-3-3 proteins. (A) His6-Xchk1 phosphorylates full-length Xwee1 on Ser-549. Bacterially expressed GST-Xwee1-WT (lanes 1 and 3) and GST-Xwee1-S549A (lanes 2 and 4) were incubated with wild-type His6-Xchk1 (lanes 1 and 2) or kinase-inactive His6-Xchk1-N135A (lanes 3 and 4). The kinase reactions were subjected to SDS-PAGE and analyzed by autoradiography (top) and Coomassie blue staining (bottom). (B) His6-Xchk1 phosphorylates a C-terminal peptide derived from Xwee1. Bacterially expressed GST-Cdc25(254-316)-WT (lane 1), GST-Xwee1(475-555)-WT (lanes 2 and 4), and GST-Xwee1(475-555)-S549A (lanes 3 and 5) were incubated with His6-Xchk1 (lanes 1–3) or His6-Xchk1-N135A (lanes 4 and 5). The kinase reactions were processed as in A. (C) Interphase egg extracts were subjected to immunoprecipitation with control antibodies (lane 1) or anti-Xchk1 antibodies (lane 2). The resulting immunoprecipitates were assayed for kinase activity toward GST-Xwee1(475-555)-WT (top) or GST-Cdc25(254-316)-WT (bottom). The kinase reactions were then processed for SDS-PAGE and autoradiography. (D) Ser-549 is a physiological site of phosphorylation in interphase Xenopus egg extracts. His6-GST-Xwee1 (left) and His6-GST-Xwee1-S549A (right) were labeled with 32P and subjected to two-dimensional tryptic phosphopeptide mapping as described in MATERIALS AND METHODS. The position of the radioactive spot that is missing from the map of the S549A mutant is marked with an arrow. The mobility of this phosphopeptide was confirmed by mixing the tryptic digests of the wild-type and S549A proteins. The origin is indicated by a dot in the lower left-hand corner. (E) GST-Xwee1(475-555)-WT (lanes 1–3) and GST-Xwee1(475-555)-S549A (lane 4), both of which were immobilized on glutathione agarose, were treated with no kinase (lane 1), wild-type His6-Xchk1 (lanes 2 and 4), or His6-Xchk1-N135A (lane 3) in the presence of ATP. Next, His6–14-3-3ε was added to all four incubations. After extensive washing, bound proteins were eluted with 50 mM glutathione, and the eluates were analyzed by immunoblotting with anti-Xwee1 (top) or anti-14-3-3ε (bottom) antibodies.